Hepatitis C disease severity in living versus deceased donor liver transplant recipients: an extended observation study
- PMID: 24677192
- PMCID: PMC4118586
- DOI: 10.1002/hep.26920
Hepatitis C disease severity in living versus deceased donor liver transplant recipients: an extended observation study
Abstract
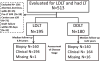
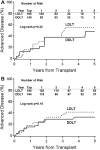
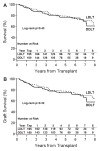
Donor factors influence hepatitis C virus (HCV) disease severity in liver transplant (LT) recipients. Living donors, because they are typically young and have short cold ischemic times, may be advantageous for HCV-infected patients. Among HCV-infected patients in the Adult-to-Adult Living Donor Liver Transplantation Cohort Study (A2ALL) surviving >90 days and followed for a median 4.7 years, advanced fibrosis (Ishak stage ≥3) and graft loss were determined. The 5-year cumulative risk of advanced fibrosis was 44% and 37% in living donor LT (LDLT) and deceased donor LT (DDLT) patients (P = 0.16), respectively. Aspartate aminotransferase (AST) activity at LT (hazard ratio [HR] = 1.38 for doubling of AST, P = 0.005) and biliary strictures (HR = 2.68, P = 0.0001) were associated with advanced fibrosis, but LDLT was not (HR = 1.11, 95% confidence interval [CI] 0.73-1.69, P = 0.63). The 5-year unadjusted patient and graft survival probabilities were 79% and 78% in LDLT, and 77% and 75% in DDLT (P = 0.43 and 0.32), with 27% and 20% of LDLT and DDLT graft losses due to HCV (P = 0.45). Biliary strictures (HR = 2.25, P = 0.0006), creatinine at LT (HR = 1.74 for doubling of creatinine, P = 0.0004), and AST at LT (HR = 1.36 for doubling of AST, P = 0.004) were associated with graft loss, but LDLT was not (HR = 0.76, 95% CI: 0.49-1.18, P = 0.23).
Conclusion: Donor type does not affect the probability of advanced fibrosis or patient and graft survival in HCV-infected recipients. Thus, while LDLT offers the advantage of shorter wait times, there is no apparent benefit for HCV disease progression. Biliary strictures have a negative effect on HCV fibrosis severity and graft survival, and a high AST at LT may be an important predictor of fibrosis risk post-LT.
© 2014 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
Potential conflict of interest: Dr. Saab consults for, advises, is on the speakers' bureau for, and owns stock in Bristol-Myers Squibb and Gilead. He consults for, advises, and is on the speakers' bureau for Genentech, Merck, and Vertex. He consults for and advises Janssen and Boehringer Ingelheim. Dr. Brown consults for and received grants from Salix. He received grants from Gilead, Bristol-Myers Squibb, Roche, Vertex, and Schering Dr. Kulik received grants from Gilead.
Figures
References
-
- Thuluvath P, Guidinger M, Fung J, Johnson L, Rayhill S, Pelletier S. Liver transplantation in the United States, 1999-2008. Am J Transplant. 2010;10(4 Pt 2):1003–1019. - PubMed
-
- Berenguer M, Schuppan D. Progression of liver fibrosis in posttransplant hepatitis C: Mechanisms, assessment and treatment. J Hepatol. 2013;58:1028–1041. - PubMed
-
- Selzner N, Girgrah N, Lilly L, Guindi M, Selzner M, Therapondos G, et al. The difference in the fibrosis progression of recurrent hepatitis C after live donor liver transplantation versus deceased donor liver transplantation is attributable to the difference in donor age. Liver Transpl. 2008;14:1778–1786. - PubMed
-
- Garcia-Retortillo M, Forns X, Llovet J, Navasa M, Feliu A, Massaguer A, et al. Hepatitis C recurrence is more severe after living donor compared to cadaveric liver transplantation. Hepatology. 2004;40:699–707. - PubMed
-
- Schmeding M, Neumann UP, Puhl G, Bahra M, Neuhaus R, Neuhaus P. Hepatitis C recurrence and fibrosis progression are not increased after living donor liver transplantation: a single-center study of 289 patients. Liver Transpl. 2007;13:687–692. - PubMed
Publication types
MeSH terms
Grants and funding
- DK62505/DK/NIDDK NIH HHS/United States
- U01 DK062536/DK/NIDDK NIH HHS/United States
- DK62484/DK/NIDDK NIH HHS/United States
- U01-DK62536/DK/NIDDK NIH HHS/United States
- DK62444/DK/NIDDK NIH HHS/United States
- U01-DK62505/DK/NIDDK NIH HHS/United States
- U01 DK062498/DK/NIDDK NIH HHS/United States
- U01 DK062484/DK/NIDDK NIH HHS/United States
- DK62531/DK/NIDDK NIH HHS/United States
- U01-DK62483/DK/NIDDK NIH HHS/United States
- U01 DK062483/DK/NIDDK NIH HHS/United States
- U01 DK062467/DK/NIDDK NIH HHS/United States
- DK62498/DK/NIDDK NIH HHS/United States
- U01 DK062496/DK/NIDDK NIH HHS/United States
- U01-DK62444/DK/NIDDK NIH HHS/United States
- P30 DK026743/DK/NIDDK NIH HHS/United States
- U01-DK62467/DK/NIDDK NIH HHS/United States
- U01 DK062531/DK/NIDDK NIH HHS/United States
- U01 DK062444/DK/NIDDK NIH HHS/United States
- U01-DK62531/DK/NIDDK NIH HHS/United States
- U01-DK62484/DK/NIDDK NIH HHS/United States
- U01 DK062505/DK/NIDDK NIH HHS/United States
- U01-DK62498/DK/NIDDK NIH HHS/United States
- DK62536/DK/NIDDK NIH HHS/United States
- U01-DK62494/DK/NIDDK NIH HHS/United States
- DK62494/DK/NIDDK NIH HHS/United States
- DK62467/DK/NIDDK NIH HHS/United States
- DK62483/DK/NIDDK NIH HHS/United States
- DK62496/DK/NIDDK NIH HHS/United States
- U01-DK62496/DK/NIDDK NIH HHS/United States
- U01 DK062494/DK/NIDDK NIH HHS/United States
