The minus-end actin capping protein, UNC-94/tropomodulin, regulates development of the Caenorhabditis elegans intestine
- PMID: 24677443
- PMCID: PMC4166613
- DOI: 10.1002/dvdy.24118
The minus-end actin capping protein, UNC-94/tropomodulin, regulates development of the Caenorhabditis elegans intestine
Abstract
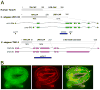
Background: Tropomodulins are actin-capping proteins that regulate the stability of the slow-growing, minus-ends of actin filaments. The C. elegans tropomodulin homolog, UNC-94, has sequence and functional similarity to vertebrate tropomodulins. We investigated the role of UNC-94 in C. elegans intestinal morphogenesis.
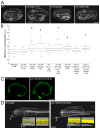
Results: In the embryonic C. elegans intestine, UNC-94 localizes to the terminal web, an actin- and intermediate filament-rich structure that underlies the apical membrane. Loss of UNC-94 function results in areas of flattened intestinal lumen. In worms homozygous for the strong loss-of-function allele, unc-94(tm724), the terminal web is thinner and the amount of F-actin is reduced, pointing to a role for UNC-94 in regulating the structure of the terminal web. The non-muscle myosin, NMY-1, also localizes to the terminal web, and we present evidence that increasing actomyosin contractility by depleting the myosin phosphatase regulatory subunit, mel-11, can rescue the flattened lumen phenotype of unc-94 mutants.
Conclusions: The data support a model in which minus-end actin capping by UNC-94 promotes proper F-actin structure and contraction in the terminal web, yielding proper shape of the intestinal lumen. This establishes a new role for a tropomodulin in regulating lumen shape during tubulogenesis.
Keywords: actomyosin contractility; terminal web; tubulogenesis.
© 2014 Wiley Periodicals, Inc.
Figures




References
-
- Buechner M. Tubes and the single C. elegans excretory cell. Trends Cell Biol. 2002;10:479–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

