Structure and function of LGR5: an enigmatic G-protein coupled receptor marking stem cells
- PMID: 24677446
- PMCID: PMC4005707
- DOI: 10.1002/pro.2446
Structure and function of LGR5: an enigmatic G-protein coupled receptor marking stem cells
Abstract
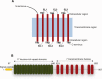
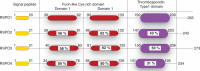
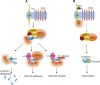
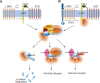
G-protein coupled receptors (GPCRs) are an important class of membrane protein that transmit extracellular signals invoked by sensing molecules such as hormones and neurotransmitters. GPCR dysfunction is implicated in many diseases and hence these proteins are of great interest to academia and the pharmaceutical industry. Leucine-rich repeat-containing GPCRs contain a characteristic extracellular domain that is an important modulator of intracellular signaling. One member of this class is the leucine-rich repeat-containing G-protein-coupled receptor 5 (LGR5), a stem cell marker in intestinal crypts, and mammary glands. LGR5 modulates Wnt signaling in the presence of the ligand R-spondin (RSPO). The mechanism of activation of LGR5 by RSPO is not understood, nor is the intracellular signaling mechanism known. Recently reported structures of the extracellular domain of LGR5 bound to RSPO reveal a horseshoe-shaped architecture made up of consecutive leucine-rich repeats, with RSPO bound on the concave surface. This review discusses the discovery of LGR5 and the impact it is having on our understanding of stem cell and cancer biology of the colon. In addition, it covers functional relationships suggested by sequence homology and structural analyses, as well as some intriguing conundrums with respect to the involvement of LGR5 in Wnt signaling.
Keywords: GPCR; LGR5; RSPO; Wnt signaling; colon cancer; stem cells.
© 2014 The Protein Society.
Figures




References
-
- Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–1272. - PubMed
-
- Ji TH, Grossmann M, Ji I. G protein-coupled receptors. I. Diversity of receptor-ligand interactions. J Biol Chem. 1998;273:17299–17302. - PubMed
-
- Lundstrom K. Latest development in drug discovery on G protein-coupled receptors. Curr Protein Pept Sci. 2006;7:465–470. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

