Erythropoiesis from human embryonic stem cells through erythropoietin-independent AKT signaling
- PMID: 24677652
- PMCID: PMC4037366
- DOI: 10.1002/stem.1677
Erythropoiesis from human embryonic stem cells through erythropoietin-independent AKT signaling
Abstract
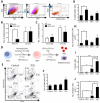
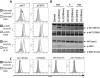
Unlimited self renewal capacity and differentiation potential make human pluripotent stem cells (PSC) a promising source for the ex vivo manufacture of red blood cells (RBCs) for safe transfusion. Current methods to induce erythropoiesis from PSC suffer from low yields of RBCs, most of which are immature and contain embryonic and fetal rather than adult hemoglobins. We have previously shown that homodimerization of the intracellular component of MPL (ic-MPL) induces erythropoiesis from human cord blood progenitors. The goal of this study was to investigate the potential of ic-MPL dimerization to induce erythropoiesis from human embryonic stem cells (hESCs) and to identify the signaling pathways activated by this strategy. We present here the evidence that ic-MPL dimerization induces erythropoietin (EPO)-independent erythroid differentiation from hESC by inducing the generation of erythroid progenitors and by promoting more efficient erythroid maturation with increased RBC enucleation as well as increased gamma:epsilon globin ratio and production of beta-globin protein. ic-MPL dimerization is significantly more potent than EPO in inducing erythropoiesis, and its effect is additive to EPO. Signaling studies show that dimerization of ic-MPL, unlike stimulation of the wild type MPL receptor, activates AKT in the absence of JAK2/STAT5 signaling. AKT activation upregulates GATA-1 and FOXO3 transcriptional pathways with resulting inhibition of apoptosis, modulation of cell cycle, and enhanced maturation of erythroid cells. These findings open up potential new targets for the generation of therapeutically relevant RBC products from hPSC.
Keywords: AKT; Erythropoiesis; Erythropoietin; GATA-1; Human embryonic stem cells; MPL.
© 2014 AlphaMed Press.
Figures
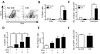


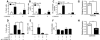
References
-
- Carson J, Grossman B, Kleinman S, et al. Red Blood Cell Transfusion: A Clinical Practice Guideline From the AABB. Annals of Internal Medicine. 2012;157:49–U95. - PubMed
-
- Olivier E, Qiu C, Velho M, et al. Large-scale production of embryonic red blood cells from human embryonic stem cells. Experimental Hematology. 2006;34:1635–1642. - PubMed
-
- Kendall RG. Erythropoietin. Clin Lab Haematol. 2001;23:71–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

