Clinical outcomes after utilizing surviving sepsis campaign in children with septic shock and prognostic value of initial plasma NT-proBNP
- PMID: 24678148
- PMCID: PMC3943130
- DOI: 10.4103/0972-5229.126075
Clinical outcomes after utilizing surviving sepsis campaign in children with septic shock and prognostic value of initial plasma NT-proBNP
Abstract
Background and objective: The surviving sepsis campaign treatment guideline (SSC) implementation is associated with improved outcome in adults with severe sepsis. The effect on outcome of pediatric sepsis is less clear.
Purpose: To determine the clinical outcomes of SSC implementation and to investigate the prognostic value of initial plasma NT-proBNP and procalcitonin in children.
Materials and methods: Infants and children (aged 1month/0-15 years with severe sepsis or septic shock) were prospectively enrolled and treated according to the guidelines. Initial blood drawn was saved for NT-pro-BNP, procalcitonin measurements and clinical data were also recorded.
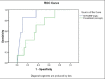
Results: A total of 47 subjects were recruited. Since the application of the SSC, our mortality rate had significantly decreased from 42-19% (P = 0.003) as compared to the data in the previous 3 years. Clinical factors that significantly increased the mortality rate were: Initial central venous oxygen saturation < 7 0% after fluid resuscitation [odds ratio (OR) = 23.3; 95% confidence interval (CI) 3.7-143; P = 0.001], and initial albumin level (≤ 3 g/dl, OR = 6.7; 95% CI 1.2-37.5, P = 0.03). There was asignificant difference between the initial NT-proBNP levels between survivors and non survivors, (6280.3 ± 9597 ng/L, P < 0.001), but not for procalcitonin (12.7 ± 24.8, 29.3 ± 46 μg/L, P = 0.1), respectively. An initial NT-proBNP level of more than 11,200 pg/ml predicted Pediatric Intensive Care Unit (PICU) mortality with a sensitivity of 85.7% and a specificity of 90%.
Conclusions: A modified SSC for severe sepsis and septic shock significantly reduced the mortality rate in our PICU. High initial NT-ProBNP level was associated with mortality.
Keywords: Biomarker; SSC guideline; decrease mortality; mortality; pediatric sepsis; severe sepsis; surviving sepsis campaign.
Conflict of interest statement
Figures



Similar articles
-
Procalcitonin and N-Terminal Pro-B-Type Natriuretic Peptide for Prognosis in Septic Acute Kidney Injury Patients Receiving Renal Replacement Therapy.Blood Purif. 2019;48(3):262-271. doi: 10.1159/000501388. Epub 2019 Jul 16. Blood Purif. 2019. PMID: 31311006
-
Predictive value of N-terminal pro-brain natriuretic peptide in severe sepsis and septic shock.Crit Care Med. 2007 May;35(5):1277-83. doi: 10.1097/01.CCM.0000261893.72811.0F. Crit Care Med. 2007. PMID: 17414731
-
[Prognostic value of plasma N-terminal pro-B-type natriuretic peptide in patients with severe sepsis and septic shock].Sichuan Da Xue Xue Bao Yi Xue Ban. 2011 May;42(3):369-73. Sichuan Da Xue Xue Bao Yi Xue Ban. 2011. PMID: 21827001 Chinese.
-
Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012.Crit Care Med. 2013 Feb;41(2):580-637. doi: 10.1097/CCM.0b013e31827e83af. Crit Care Med. 2013. PMID: 23353941
-
Natriuretic Peptides to Predict Short-Term Mortality in Patients With Sepsis: A Systematic Review and Meta-analysis.Mayo Clin Proc Innov Qual Outcomes. 2020 Jan 8;4(1):50-64. doi: 10.1016/j.mayocpiqo.2019.10.008. eCollection 2020 Feb. Mayo Clin Proc Innov Qual Outcomes. 2020. PMID: 32055771 Free PMC article. Review.
Cited by
-
Diagnostic Accuracy of NT-ProBNP for Heart Failure with Sepsis in Patients Younger than 18 Years.PLoS One. 2016 Jan 26;11(1):e0147930. doi: 10.1371/journal.pone.0147930. eCollection 2016. PLoS One. 2016. PMID: 26812689 Free PMC article.
-
Cardiac Biomarkers in Pediatrics: An Undervalued Resource.Clin Chem. 2021 Jul 6;67(7):947-958. doi: 10.1093/clinchem/hvab063. Clin Chem. 2021. PMID: 34125147 Free PMC article. Review.
-
Assessment of early goal-directed therapy guideline adherence: Balancing clinical importance and feasibility.PLoS One. 2019 Mar 15;14(3):e0213802. doi: 10.1371/journal.pone.0213802. eCollection 2019. PLoS One. 2019. PMID: 30875402 Free PMC article.
-
Association of Single-Nucleotide Polymorphisms of C-Reactive Protein Gene with Susceptibility to Infantile Sepsis in Southern China.Med Sci Monit. 2018 Jan 30;24:590-595. doi: 10.12659/msm.908602. Med Sci Monit. 2018. PMID: 29379005 Free PMC article.
-
Cardiovascular Dysfunction Criteria in Critically Ill Children: The PODIUM Consensus Conference.Pediatrics. 2022 Jan 1;149(1 Suppl 1):S39-S47. doi: 10.1542/peds.2021-052888F. Pediatrics. 2022. PMID: 34970677 Free PMC article.
References
-
- Kissoon N, Argent A, Devictor D, Madden MA, Singhi S, van der Voort E, et al. World federation of pediatric intensive and critical care societies-its global agenda. Pediatr Crit Care Med. 2009;10:597–600. - PubMed
-
- Oliveira C, Nogueira F, Oliveira D, Gottschald AF, Moura JD, Shibata AR, et al. Time-and fluid sensitive resuscitation for hemodynamic support of children in septic shock: Barriers to the implementation of the American College of Critical Care Medicine/Pediatric Advanced Life Support Guidelines in a pediatric intensive care unit in a developing world. Pediatr Emerg Care. 2008;24:810–5. - PubMed
-
- Wolfer A, Silvani P, Musicco M, Antonelli M, Salvo I Italian Pediatric Sepsis Study (SISPe) group. Incidence of and mortality due to sepsis, severe sepsis and septic shock in Italian Pediatric Intensive Care units: A prospective national survey. Intensive Care Med. 2008;34:1690–7. - PubMed
-
- Samransamruajkit R, Hiranrat T, Prapphal N, Sritippayawan S, Deerojanawong J, Poovorawan Y. Levels of protein C activity and clinical factors in early phase of pediatric septic shock may be associated with the risk of death. Shock. 2007;28:518–23. - PubMed
-
- Carcillo JA, Fields A American College of Critical Care Medicine Task Force Committee Members. Clinical practice parameters for hemodynamic support of pediatric and neonatal patients in septic shock. Crit Care Med. 2002;30:1365–78. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
