Hepatitis C virus: Screening, diagnosis, and interpretation of laboratory assays
- PMID: 24678168
- PMCID: PMC3943138
- DOI: 10.4103/0973-6247.126683
Hepatitis C virus: Screening, diagnosis, and interpretation of laboratory assays
Abstract
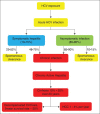
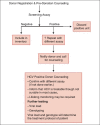
An estimated 3% of the world population is infected with Hepatitis C virus (HCV), a hepatotropic RNA virus, transmitted primarily via the blood route. The major modes of transmission of the virus include injection drug use, unsafe injection practices, blood transfusion etc. HCV causes chronic hepatitis in about 80% of those infected by it. The mainstay in diagnosing infection with HCV is to initially screen high risk groups for antibodies to HCV (anti-HCV). The inclusion of serum to cut-off ratio (S/CO) in recent guidelines is helpful in deciding the supplemental assay to be used to confirm initially reactive screening results. Nucleic acid amplification tests (NAT) are used as confirmatory tools, and also to determine viral load prior to initiating treatment. Quantitative NAT has replaced qualitative assays. Genotyping is an important tool in clinical management to predict the likelihood of response and determine the optimal duration of therapy. The impact of this infection has begun to emerge in India. The problem of professional blood donation despite an existing law against it, and flourishing unsafe injection practices, are potential sources for the spread of hepatitis C in our country. All health care practitioners need to understand how to establish or exclude a diagnosis of HCV infection and to interpret the tests correctly. In the absence of a preventive or therapeutic vaccine, and also of post-exposure prophylaxis against the virus, it is imperative to diagnose infection by HCV so as to prevent hepatic insult and the ensuing complications that follow, including primary hepatocellular carcinoma (HCC). This review aims to help blood bank staff regarding options for diagnosis and management of donors positive for HCV.
Keywords: Blood borne virus; hepatitis C virus diagnosis; nucleic acid test.
Conflict of interest statement
Figures
References
-
- Forman MS, Valsamakis A. Hepatitis C virus. In: Versalovic J, Carrol KC, Funke G, Jorgensen JH, Landry ML, Warrock DW, editors. Murray's Manual of Clinical Microbiology. 10th ed. Washington: American Society of Microbiology Press; 2011. pp. 1437–55.
-
- Alter MJ, Seeff LB, Bacon BR, Thomas DL, Rigsby MO, Di Bisceglie AM. Testing for hepatitis C virus infection should be routine for persons at increased risk for infection. Ann Intern Med. 2004;141:715–7. - PubMed
-
- Feinstone SM, Kapikian AZ, Purcell RH, Alter HJ, Holland PV. Transfusion-associated hepatitis not due to viral hepatitis type A or B. N Engl J Med. 1975;292:767–70. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources