Phytochemicals as Anticancer and Chemopreventive Topoisomerase II Poisons
- PMID: 24678287
- PMCID: PMC3963363
- DOI: 10.1007/s11101-013-9291-7
Phytochemicals as Anticancer and Chemopreventive Topoisomerase II Poisons
Abstract
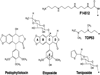
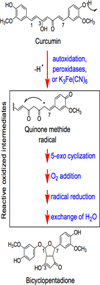
Phytochemicals are a rich source of anticancer drugs and chemopreventive agents. Several of these chemicals appear to exert at least some of their effects through interactions with topoisomerase II, an essential enzyme that regulates DNA supercoiling and removes knots and tangles from the genome. Topoisomerase II-active phytochemicals function by stabilizing covalent protein-cleaved DNA complexes that are intermediates in the catalytic cycle of the enzyme. As a result, these compounds convert topoisomerase II to a cellular toxin that fragments the genome. Because of their mode of action, they are referred to as topoisomerase II poisons as opposed to catalytic inhibitors. The first sections of this article discuss DNA topology, the catalytic cycle of topoisomerase II, and the two mechanisms (interfacial vs. covalent) by which different classes of topoisomerase II poisons alter enzyme activity. Subsequent sections discuss the effects of several phytochemicals on the type II enzyme, including demethyl-epipodophyllotoxins (semisynthetic anticancer drugs) as well as flavones, flavonols, isoflavones, catechins, isothiocyanates, and curcumin (dietary chemopreventive agents). Finally, the leukemogenic potential of topoisomerase II-targeted phytochemicals is described.
Keywords: Bioflavonoids; Catechins; Curcumin; Demethyl-epipodophyllotoxins; Isothiocyanates.
Figures







References
-
- Adlercreutz H, Markkanen H, Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet. 1993;342:1209–1210. - PubMed
-
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. - PubMed
-
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. - PubMed
-
- Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, Tharakan ST, Misra K, Priyadarsini IK, Rajasekharan KN, Aggarwal BB. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem Pharmacol. 2008;76:1590–1611. - PubMed
-
- Andoh T, Ishida R. Catalytic inhibitors of DNA topoisomerase II. Biochimica et Biophysica Acta. 1998;1400:155–171. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
