Ginseng ginsenoside pharmacology in the nervous system: involvement in the regulation of ion channels and receptors
- PMID: 24678300
- PMCID: PMC3958645
- DOI: 10.3389/fphys.2014.00098
Ginseng ginsenoside pharmacology in the nervous system: involvement in the regulation of ion channels and receptors
Abstract
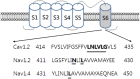
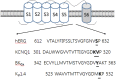
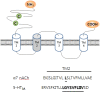
Ginseng, the root of Panax ginseng C.A. Meyer, is one of the oldest traditional medicines and is thought to be a tonic. It has been claimed that ginseng may improve vitality and health. Recent studies have advanced ginseng pharmacology and shown that ginseng has various pharmacological effects in the nervous system. Ginsenosides, steroid glycosides extracted from ginseng, were one of the first class of biologically active plant glycosides identified. The diverse pharmacological effects of ginsenosides have been investigated through the regulation of various types of ion channels and receptors in neuronal cells and heterologous expression systems. Ginsenoside Rg3 regulates voltage-gated ion channels such as Ca(2+), K(+), and Na(+) channels, and ligand-gated ion channels such as GABAA, 5-HT3, nicotinic acetylcholine, and N-methyl-D-aspartate (NMDA) receptors through interactions with various sites including channel blocker binding sites, toxin-binding sites, channel gating regions, and allosteric channel regulator binding sites when the respective ion channels or receptors are stimulated with depolarization or ligand treatment. Treatment with ginsenoside Rg3 has been found to stabilize excitable cells by blocking influxes of cations such as Ca(2+) and Na(+), or by enhancing Cl(-) influx. The aim of this review is to present recent findings on the pharmacological functions of the ginsenosides through the interactions with ion channels and receptors. This review will detail the pharmacological applications of ginsenosides as neuroprotective drugs that target ion channels and ligand-gated ion channels.
Keywords: ginseng; ginsenosides; interaction site(s); ion channels and receptors; nervous system.
Figures
References
-
- Bai C. X., Takahashi K., Masumiya H., Sawanobori T., Furukawa T. (2004). Nitric oxide-dependent modulation of the delayed rectifier K+ current and the L-type Ca2+ current by ginsenoside Re, an ingredient of Panax ginseng, in guinea-pig cardiomyocytes. Br. J. Pharmacol. 142, 567–575 10.1038/sj.bjp.0705814 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

