The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles
- PMID: 24678386
- PMCID: PMC3967015
- DOI: 10.3402/jev.v3.23111
The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles
Abstract
Background: Extracellular vesicles (EV), the collective term for vesicles released from cells, consist of vesicle species ranging in size from 30 nm to 5 µm in diameter. These vesicles are most commonly isolated by differential centrifugations, which pellets particles based on their differential movement through the liquid medium in which they are immersed. Multiple parameters, including the utilization of different rotor types, can influence the yield and purity of isolated vesicles; however, the understanding of how these factors affect is limited.
Materials and methods: Here, we compare the influence of multiple centrifugation parameters, including the use of swinging bucket and fixed angle rotors, as well as different centrifugation times, for the isolation of the smallest EVs, "exosomes." In particular, we determine the yields of exosomal RNA and protein, as well as the nature of the isolated vesicles and possible protein contamination with methods such as electron microscopy, western blot and flow cytometry.
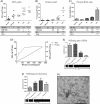
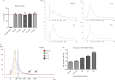
Results: Our results show that application of a specific g-force or rotation speed by itself does not predict the ability of pelleting exosomes, and that prolonged centrifugation times can achieve greater yields of exosomal RNA and protein, whereas very long centrifugation times result in excessive protein concentrations in the exosome pellet.
Conclusion: In conclusion, rotor type, g-force and centrifugation times significantly influence exosome yield during centrifugation-based isolation procedures, and current commonly recommended isolation protocols may not be fully optimized for yield and purity of exosomes.
Keywords: exosomes; extracellular vesicles; isolation protocol; rotor; ultracentrifugation.
Figures



References
-
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. - PubMed
-
- Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–20. - PubMed
-
- van der Pol E, Boing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676–705. - PubMed
-
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

