Postpartum breast involution reveals regression of secretory lobules mediated by tissue-remodeling
- PMID: 24678808
- PMCID: PMC4053254
- DOI: 10.1186/bcr3633
Postpartum breast involution reveals regression of secretory lobules mediated by tissue-remodeling
Abstract
Introduction: A postpartum diagnosis of breast cancer is an independent predictor of metastases, however the reason is unknown. In rodents, the window of postpartum mammary gland involution promotes tumor progression, suggesting a role for breast involution in the poor prognosis of human postpartum breast cancers. Rodent mammary gland involution is characterized by the programmed elimination of the secretory lobules laid down in preparation for lactation. This tissue involution process involves massive epithelial cell death, stromal remodeling, and immune cell infiltration with similarities to microenvironments present during wound healing and tumor progression. Here, we characterize breast tissue from premenopausal women with known reproductive histories to determine the extent, duration and cellular mechanisms of postpartum lobular involution in women.
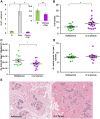
Methods: Adjacent normal breast tissues from premenopausal women (n = 183) aged 20 to 45 years, grouped by reproductive categories of nulliparous, pregnant and lactating, and by time since last delivery were evaluated histologically and by special stain for lobular area, lobular type composition, apoptosis and immune cell infiltration using computer assisted quantitative methods.
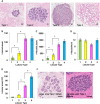
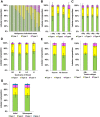
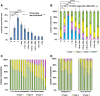
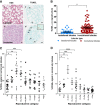
Results: Human nulliparous glands were composed dominantly of small (approximately 10 acini per lobule) and medium (approximately 35 acini per lobule) sized lobules. With pregnancy and lactation, a >10 fold increase in breast epithelial area was observed compared to nulliparous cases, and lactating glands were dominated by mature lobules (>100 acini per lobule) with secretory morphology. Significant losses in mammary epithelial area and mature lobule phenotypes were observed within 12 months postpartum. By 18 months postpartum, lobular area content and lobule composition were indistinguishable from nulliparous cases, data consistent with postpartum involution facilitating regression of the secretory lobules developed in preparation for lactation. Analyses of apoptosis and immune cell infiltrate confirmed that human postpartum breast involution is characterized by wound healing-like tissue remodeling programs that occur within a narrowed time frame.
Conclusions: Human postpartum breast involution is a dominant tissue-remodeling process that returns the total lobular area of the gland to a level essentially indistinguishable from the nulliparous gland. Further research is warranted to determine whether the normal physiologic process of postpartum involution contributes to the poor prognosis of postpartum breast cancer.
Figures
References
-
- Albrektsen G, Heuch I, Tretli S, Kvale G. Breast cancer incidence before age 55 in relation to parity and age at first and last births: a prospective study of one million Norwegian women. Epidemiology. 1994;5:604–611. - PubMed
-
- Lambe M, Hsieh C, Trichopoulos D, Ekbom A, Pavia M, Adami HO. Transient increase in the risk of breast cancer after giving birth. N Engl J Med. 1994;331:5–9. - PubMed
-
- Chie WC, Hsieh C, Newcomb PA, Longnecker MP, Mittendorf R, Greenberg ER, Clapp RW, Burke KP, Titus-Ernstoff L, Trentham-Dietz A, MacMahon B. Age at any full-term pregnancy and breast cancer risk. Am J Epidemiol. 2000;151:715–722. - PubMed
-
- Liu Q, Wuu J, Lambe M, Hsieh SF, Ekbom A, Hsieh CC. Transient increase in breast cancer risk after giving birth: postpartum period with the highest risk (Sweden) Cancer Causes Control. 2002;13:299–305. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

