Role of β-arrestins and arrestin domain-containing proteins in G protein-coupled receptor trafficking
- PMID: 24680432
- PMCID: PMC3971387
- DOI: 10.1016/j.ceb.2013.11.005
Role of β-arrestins and arrestin domain-containing proteins in G protein-coupled receptor trafficking
Abstract
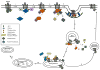
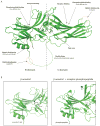
The arrestin clan can now be broadly divided into three structurally similar subgroups: the originally identified arrestins (visual and β-arrestins), the α-arrestins and a group of Vps26-related proteins. The visual and β-arrestins selectively bind to agonist-occupied phosphorylated G protein-coupled receptors (GPCRs) and inhibit GPCR coupling to heterotrimeric G proteins while the β-arrestins also function as adaptor proteins to regulate GPCR trafficking and G protein-independent signaling. The α-arrestins have also recently been implicated in regulating GPCR trafficking while Vps26 regulates retrograde trafficking. In this review, we provide an overview of the α-arrestins and β-arrestins with a focus on our current understanding of how these adaptor proteins regulate GPCR trafficking.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
References
-
- Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–482. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

