Expression analysis of Dact1 in mice using a LacZ reporter
- PMID: 24681206
- PMCID: PMC4426989
- DOI: 10.1016/j.gep.2014.03.002
Expression analysis of Dact1 in mice using a LacZ reporter
Abstract
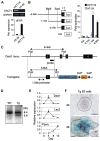
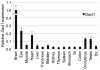
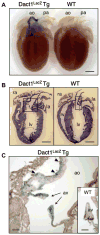
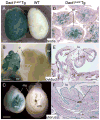
The Wnt signaling pathway is essential for cell fate decisions during embryonic development as well as for homeostasis after birth. Dapper antagonist of catenin-1 (Dact1) plays an important role during embryogenesis by regulating Wnt signaling pathways. Consequently, targeted disruption of the Dact1 gene in mice leads to perinatal lethality due to severe developmental defects involving the central nervous system, genitourinary system and distal digestive tract. However, the expression and potential function of Dact1 in other tissues during development and postnatal life have not been well studied. Here, we have generated reporter mice in which LacZ expression is driven by the Dact1 gene promoter and characterized Dact1-LacZ expression in embryos and adult tissues. Our data show that while Dact1-LacZ is expressed in multiple mesoderm- and neuroectoderm-derived tissues during development, high expression of Dact1-LacZ is restricted to a small subset of adult tissues, including the brain, eye, heart, and some reproductive organs. These results will serve as a basis for future investigation of Dact1 function in Wnt-mediated organogenesis and tissue homeostasis.
Keywords: Dact1; Embryogenesis; Homeostasis; LacZ reporter; Wnt signal.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures



References
-
- Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–477. - PubMed
-
- Cheyette BN, Waxman JS, Miller JR, Takemaru K, Sheldahl LC, Khlebtsova N, Fox EP, Earnest T, Moon RT. Dapper, a Dishevelled-associated antagonist of beta-catenin and JNK signaling, is required for notochord formation. Dev Cell. 2002;2:449–461. - PubMed
-
- Chien KR. Myocyte survival pathways and cardiomyopathy: implications for trastuzumab cardiotoxicity. Semin Oncol. 2000;27:9–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

