Defining and treating the spectrum of intermediate risk nonmuscle invasive bladder cancer
- PMID: 24681333
- PMCID: PMC4687397
- DOI: 10.1016/j.juro.2014.02.2573
Defining and treating the spectrum of intermediate risk nonmuscle invasive bladder cancer
Abstract
Purpose: Low, intermediate and high risk categories have been defined to help guide the treatment of patients with nonmuscle invasive bladder cancer (Ta, T1, CIS). However, while low and high risk disease has been well classified, the intermediate risk category has traditionally comprised a heterogeneous group that does not fit into either of these categories. As a result, many urologists remain uncertain about the categorization of patients as intermediate risk as well as the selection of the most appropriate therapeutic option for this patient population. We review the current literature and clinical practice guidelines on intermediate risk nonmuscle invasive bladder cancer and, based on our findings, provide urologists with a better understanding of this heterogeneous risk group as well as practical recommendations for the treatment of intermediate risk patients.
Materials and methods: The IBCG analyzed published clinical trials, meta-analyses and current clinical practice guidelines on intermediate risk nonmuscle invasive bladder cancer available as of September 2013. The definitions of intermediate risk, patient outcomes and guideline recommendations were considered, as were the limitations of the available literature and additional parameters that may be useful in guiding treatment decisions in intermediate risk patients.
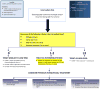
Results: Current definitions and management recommendations for intermediate risk nonmuscle invasive bladder cancer vary. The most simple and practical definition is that proposed by the IBCG and the AUA of multiple and/or recurrent low grade Ta tumors. The IBCG suggests that several factors should be considered in clinical decisions in intermediate risk disease, including number (greater than 1) and size (greater than 3 cm) of tumors, timing (recurrence within 1 year) and frequency (more than 1 per year) of recurrence, and previous treatment. In patients without these risk factors a single, immediate instillation of chemotherapy is advised. In those with 1 to 2 risk factors adjuvant intravesical therapy (intravesical chemotherapy or maintenance bacillus Calmette-Guérin) is recommended, and previous intravesical therapy should be considered when choosing between these adjuvant therapies. For those patients with 3 to 4 risk factors, maintenance bacillus Calmette-Guérin is recommended. It is also important that all intermediate risk patients are accurately risk stratified at initial diagnosis and during subsequent followup. This requires appropriate transurethral resection of the bladder tumor, vigilance to rule out carcinoma in situ or other potential high risk tumors, and review of histological material directly with the pathologist.
Conclusions: Intermediate risk disease is a heterogeneous category, and there is a paucity of independent studies comparing therapies and outcomes in subgroups of intermediate risk patients. The IBCG has proposed a management algorithm that considers tumor characteristics, timing and frequency of recurrence, and previous treatment. Subgroup analyses of intermediate risk subjects in pivotal EORTC trials and meta-analyses will be important to validate the proposed algorithm and support clear evidence-based recommendations for subgroups of intermediate risk patients.
Keywords: adjuvant; administration; chemotherapy; intravesical; mycobacterium bovis; risk; urinary bladder neoplasms.
Copyright © 2014 American Urological Association Education and Research, Inc. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Lamm D, Persad R, Colombel M, et al. Maintenance bacillus Calmette-Guérin: the standard of care for the prophylaxis and management of intermediate- and high-risk non-muscle-invasive bladder cancer. Eur Urol Suppl. 2010;9:715.
-
- Brausi MA. Challenging the EAU guidelines on non–muscle-invasive bladder cancer (NMIBC): single instillation of chemotherapy after transurethral resection of NMIBC and chemotherapy versus bacillus Calmette-Guérin in treatment of intermediate-risk tumours. Eur Urol Suppl. 2010;9:406.
-
- Witjes JA, Palou J, Soloway M, et al. Current clinical practice gaps in the treatment of intermediate- and high-risk non-muscle-invasive bladder cancer (NMIBC) with emphasis on the use of bacillus Calmette-Guérin (BCG): results of an international individual patient data survey (IPDS) BJU Int. 2013;112:742. - PMC - PubMed
-
- Konety B, Oosterlinck W, Chang S, et al. Low-grade Ta urothelial carcinoma of the bladder. In: Soloway M, Khoury S, editors. Bladder Cancer. 2. Vienna: ICUD-EAU; 2012. pp. 231–246.
-
- Babjuk M, Burger M, Zigeuner R, et al. Guidelines on Non-Muscle-Invasive Bladder Cancer (TaT1 and CIS) Arnhem, the Netherlands: European Association of Urology; 2013. [Accessed July 24, 2013]. Available at: http://www.uroweb.org/gls/pdf/05_TaT1_Bladder_Cancer_LR.pdf.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

