Amphotericin forms an extramembranous and fungicidal sterol sponge
- PMID: 24681535
- PMCID: PMC3992202
- DOI: 10.1038/nchembio.1496
Amphotericin forms an extramembranous and fungicidal sterol sponge
Abstract
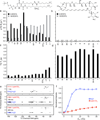
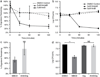
For over 50 years, amphotericin has remained the powerful but highly toxic last line of defense in treating life-threatening fungal infections in humans with minimal development of microbial resistance. Understanding how this small molecule kills yeast is thus critical for guiding development of derivatives with an improved therapeutic index and other resistance-refractory antimicrobial agents. In the widely accepted ion channel model for its mechanism of cytocidal action, amphotericin forms aggregates inside lipid bilayers that permeabilize and kill cells. In contrast, we report that amphotericin exists primarily in the form of large, extramembranous aggregates that kill yeast by extracting ergosterol from lipid bilayers. These findings reveal that extraction of a polyfunctional lipid underlies the resistance-refractory antimicrobial action of amphotericin and suggests a roadmap for separating its cytocidal and membrane-permeabilizing activities. This new mechanistic understanding is also guiding development of what are to our knowledge the first derivatives of amphotericin that kill yeast but not human cells.
Figures



Comment in
-
Antimicrobial mechanisms: a sponge against fungal infections.Nat Chem Biol. 2014 Jun;10(6):411-2. doi: 10.1038/nchembio.1518. Epub 2014 Apr 20. Nat Chem Biol. 2014. PMID: 24747527
References
-
- Cannon RD, et al. Candida albicans drug resistance - another way to cope with stress. Microbiology. 2007;153:3211–3217. - PubMed
-
- Mora-Duarte J, et al. Comparison of caspofungin and amphotericin B for invasive candidiasis. New. Engl. J. Med. 2002;347:2020–2029. - PubMed
-
- Monk BC, Goffeau A. Outwitting multidrug resistance to antifungals. Science. 2008;321:367–369. - PubMed
-
- Ermishkin LN, Kasumov KM, Potzeluyev VM. Single ionic channels induced in lipid bilayers by polyene antibiotics amphotericin-B and nystatine. Nature. 1976;262:698–699. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

