Interferon-γ induces senescence in normal human melanocytes
- PMID: 24681574
- PMCID: PMC3969336
- DOI: 10.1371/journal.pone.0093232
Interferon-γ induces senescence in normal human melanocytes
Abstract
Background: Interferon-γ (IFN-γ) plays an important role in the proceedings of vitiligo through recruiting lymphocytes to the lesional skin. However, the potential effects of IFN-γ on skin melanocytes and the subsequent contribution to the vitiligo pathogenesis are still unclear.
Objective: To investigate the effects of IFN-γ on viability and cellular functions of melanocytes.
Methods: Primary human melanocytes were treated with IFN-γ. Cell viability, apoptosis, cell cycle melanin content and intracellular reactive oxygen species (ROS) level were measured. mRNA expression was examined by real-time PCR. The release of interleukin 6 (IL-6) and heat shock protein 70 (HSP-70) was monitored by ELISA. β-galactosidase staining was utilized to evaluate melanocyte senescence.
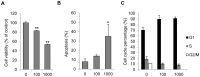
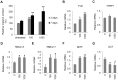
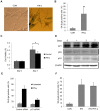
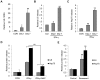
Results: Persistent IFN-γ treatment induced viability loss, apoptosis, cell cycle arrest and senescence in melanocytes. Melanocyte senescence was characterized as the changes in pigmentation and morphology, as well as the increase of β-galactosidase activity. Increase of p21Cip1/Waf1 protein was evident in melanocytes after IFN-γ treatment. IFN-γ induction of senescence was attenuated by siRNAs against p21, Janus kinase 2 (JAK2) or signal transducer and activator of transcription 1 (STAT1), but not by JAK1 siRNA nor by p53 inhibitor pifithrin-α. IFN-γ treatment increased the accumulation of intracellular ROS in melanocytes, while ROS scavenger N-acetyl cysteine (NAC) effectively inhibited IFN-γ induced p21 expression and melanocyte senescence. IL-6 and HSP-70 release was significantly induced by IFN-γ treatment, which was largely inhibited by NAC. The increase of IL-6 and HSP-70 release could also be observed in senescent melanocytes.
Conclusion: IFN-γ can induce senescence in melanocytes and consequently enhance their immuno-competency, leading to a vitiligo-prone milieu.
Conflict of interest statement
Figures


Similar articles
-
Interferon-gamma induces cellular senescence through p53-dependent DNA damage signaling in human endothelial cells.Mech Ageing Dev. 2009 Mar;130(3):179-88. doi: 10.1016/j.mad.2008.11.004. Epub 2008 Nov 21. Mech Ageing Dev. 2009. PMID: 19071156
-
TNFα-senescence initiates a STAT-dependent positive feedback loop, leading to a sustained interferon signature, DNA damage, and cytokine secretion.Aging (Albany NY). 2017 Nov 22;9(11):2411-2435. doi: 10.18632/aging.101328. Aging (Albany NY). 2017. PMID: 29176033 Free PMC article.
-
Losartan inhibits STAT1 activation and protects human glomerular mesangial cells from angiotensin II induced premature senescence.Can J Physiol Pharmacol. 2012 Jan;90(1):89-98. doi: 10.1139/y11-105. Epub 2012 Jan 4. Can J Physiol Pharmacol. 2012. PMID: 22217266
-
Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes.Exp Gerontol. 2000 Oct;35(8):927-45. doi: 10.1016/s0531-5565(00)00180-7. Exp Gerontol. 2000. PMID: 11121681 Review.
-
The human melanocyte: a model system to study the complexity of cellular aging and transformation in non-fibroblastic cells.Exp Gerontol. 2001 Aug;36(8):1265-75. doi: 10.1016/s0531-5565(01)00098-5. Exp Gerontol. 2001. PMID: 11602203 Review.
Cited by
-
Human innate immune cell crosstalk induces melanoma cell senescence.Oncoimmunology. 2020 Aug 30;9(1):1808424. doi: 10.1080/2162402X.2020.1808424. Oncoimmunology. 2020. PMID: 32939325 Free PMC article.
-
Inhibition of Fam114A1 protects melanocytes from apoptosis through higher RACK1 expression.Aging (Albany NY). 2021 Nov 27;13(22):24740-24752. doi: 10.18632/aging.203712. Epub 2021 Nov 27. Aging (Albany NY). 2021. PMID: 34837888 Free PMC article.
-
Type-2 immunity associated with type-1 related skin inflammatory diseases: friend or foe?Front Immunol. 2024 May 29;15:1405215. doi: 10.3389/fimmu.2024.1405215. eCollection 2024. Front Immunol. 2024. PMID: 38868763 Free PMC article. Review.
-
Heterogenous Differences in Cellular Senescent Phenotypes in Pre-Eclampsia and IUGR following Quantitative Assessment of Multiple Biomarkers of Senescence.Int J Mol Sci. 2023 Feb 4;24(4):3101. doi: 10.3390/ijms24043101. Int J Mol Sci. 2023. PMID: 36834513 Free PMC article.
-
Functions of heat shock proteins in pathways of the innate and adaptive immune system.J Immunol. 2014 Dec 15;193(12):5765-71. doi: 10.4049/jimmunol.1401417. J Immunol. 2014. PMID: 25480955 Free PMC article. Review.
References
-
- Taïeb A, Picardo M (2009) Clinical practice. Vitiligo. N Engl J Med 360: 160–169. - PubMed
-
- van den Boorn JG, Konijnenberg D, Dellemijn TA, van der Veen JP, Bos JD, et al. (2009) Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J Invest Dermatol 129: 2220–2232. - PubMed
-
- Steitz J, Wenzel J, Gaffal E, Tüting T (2004) Initiation and regulation of CD8+T cells recognizing melanocytic antigens in the epidermis: implications for the pathophysiology of vitiligo. Eur J Cell Biol 83: 797–803. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

