Correlative scanning-transmission electron microscopy reveals that a chimeric flavivirus is released as individual particles in secretory vesicles
- PMID: 24681578
- PMCID: PMC3969332
- DOI: 10.1371/journal.pone.0093573
Correlative scanning-transmission electron microscopy reveals that a chimeric flavivirus is released as individual particles in secretory vesicles
Abstract
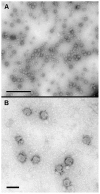
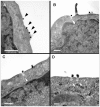
The intracellular morphogenesis of flaviviruses has been well described, but flavivirus release from the host cell remains poorly documented. We took advantage of the optimized production of an attenuated chimeric yellow fever/dengue virus for vaccine purposes to study this phenomenon by microscopic approaches. Scanning electron microscopy (SEM) showed the release of numerous viral particles at the cell surface through a short-lived process. For transmission electron microscopy (TEM) studies of the intracellular ultrastructure of the small number of cells releasing viral particles at a given time, we developed a new correlative microscopy method: CSEMTEM (for correlative scanning electron microscopy - transmission electron microscopy). CSEMTEM analysis suggested that chimeric flavivirus particles were released as individual particles, in small exocytosis vesicles, via a regulated secretory pathway. Our morphological findings provide new insight into interactions between flaviviruses and cells and demonstrate that CSEMTEM is a useful new method, complementary to SEM observations of biological events by intracellular TEM investigations.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

