5-Azacytidine treatment sensitizes tumor cells to T-cell mediated cytotoxicity and modulates NK cells in patients with myeloid malignancies
- PMID: 24681961
- PMCID: PMC3972700
- DOI: 10.1038/bcj.2014.14
5-Azacytidine treatment sensitizes tumor cells to T-cell mediated cytotoxicity and modulates NK cells in patients with myeloid malignancies
Abstract
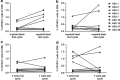
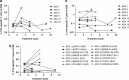
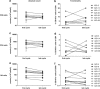
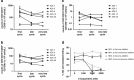
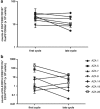
Treatment with the demethylating agent 5-Azacytidine leads to prolonged survival for patients with myelodysplastic syndrome, and the demethylation induces upregulation of cancer-testis antigens. Cancer-testis antigens are well-known targets for immune recognition in cancer, and the immune system may have a role in this treatment regimen. We show here that 5-Azacytidine treatment leads to increased T-cell recognition of tumor cells. T-cell responses against a large panel of cancer-testis antigens were detected before treatment, and these responses were further induced upon initiation of treatment. These characteristics point to an ideal combination of 5-Azacytidine and immune therapy to preferentially boost T-cell responses against cancer-testis antigens. To initiate such combination therapy, essential knowledge is required about the general immune modulatory effect of 5-Azacytidine. We therefore examined potential treatment effects on both immune stimulatory (CD8 and CD4 T cells and Natural Killer (NK) cells) and immune inhibitory cell subsets (myeloid-derived suppressor cells and regulatory T cells). We observed a minor decrease and modulation of NK cells, but for all other populations no effects could be detected. Together, these data support a strategy for combining 5-Azacytidine treatment with immune therapy for potential clinical benefit.
Figures
References
-
- Silverman LR, McKenzie DR, Peterson BL, Holland JF, Backstrom JT, Beach CL, et al. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J Clin Oncol. 2006;24:3895–3903. - PubMed
-
- Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–2440. - PubMed
-
- Fenaux P, Mufti GJ, Hellström-Lindberg E, Santini V, Gattermann N, Germing U, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28:562–569. - PubMed
-
- Adès L, Sekeres MA, Wolfromm A, Teichman ML, Tiu RV, Itzykson R, et al. Predictive factors of response and survival among chronic myelomonocytic leukemia patients treated with azacitidine. Leuk Res. 2013;37:609–613. - PubMed
-
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

