Lowering GTP level increases survival of amino acid starvation but slows growth rate for Bacillus subtilis cells lacking (p)ppGpp
- PMID: 24682323
- PMCID: PMC4010990
- DOI: 10.1128/JB.01471-14
Lowering GTP level increases survival of amino acid starvation but slows growth rate for Bacillus subtilis cells lacking (p)ppGpp
Abstract
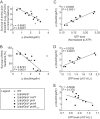
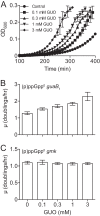
Bacterial cells sense external nutrient availability to regulate macromolecular synthesis and consequently their growth. In the Gram-positive bacterium Bacillus subtilis, the starvation-inducible nucleotide (p)ppGpp negatively regulates GTP levels, both to resist nutritional stress and to maintain GTP homeostasis during growth. Here, we quantitatively investigated the relationship between GTP level, survival of amino acid starvation, and growth rate when GTP synthesis is uncoupled from its major homeostatic regulator, (p)ppGpp. We analyzed growth and nucleotide levels in cells that lack (p)ppGpp and found that their survival of treatment with a nonfunctional amino acid analog negatively correlates with both growth rate and GTP level. Manipulation of GTP levels modulates the exponential growth rate of these cells in a positive dose-dependent manner, such that increasing the GTP level increases growth rate. However, accumulation of GTP levels above a threshold inhibits growth, suggesting a toxic effect. Strikingly, adenine counteracts GTP stress by preventing GTP accumulation in cells lacking (p)ppGpp. Our results emphasize the importance of maintaining appropriate levels of GTP to maximize growth: cells can survive amino acid starvation by decreasing GTP level, which comes at a cost to growth, while (p)ppGpp enables rapid adjustment to nutritional stress by adjusting GTP level, thus maximizing fitness.
Figures





References
-
- Bremer H, Dennis PP. 7 October 2008. Chapter 5.2.3, Modulation of chemical composition and other parameters of the cell at different exponential growth rates. In Böck A, Curtiss R, III, Kaper JB, Karp PD, Neidhardt FC, Nyström T, Slauch JM, Squires CL, Ussery D. (ed), EcoSal—Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC. 10.1128/ecosal.5.2.4 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

