Mesenchymal precursor cells as adjunctive therapy in recipients of contemporary left ventricular assist devices
- PMID: 24682346
- PMCID: PMC4243683
- DOI: 10.1161/CIRCULATIONAHA.113.007412
Mesenchymal precursor cells as adjunctive therapy in recipients of contemporary left ventricular assist devices
Abstract
Background: Allogeneic mesenchymal precursor cells (MPCs) injected during left ventricular assist device (LVAD) implantation may contribute to myocardial recovery. This trial explores the safety and efficacy of this strategy.
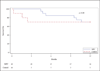
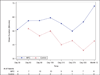
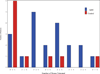
Methods and results: In this multicenter, double-blind, sham-procedure controlled trial, 30 patients were randomized (2:1) to intramyocardial injection of 25 million MPCs or medium during LVAD implantation. The primary safety end point was incidence of infectious myocarditis, myocardial rupture, neoplasm, hypersensitivity reaction, and immune sensitization (90 days after randomization). Key efficacy end points were functional status and ventricular function while temporarily weaned from LVAD support (90 days after randomization). Patients were followed up until transplant or 12 months after randomization, whichever came first. Mean age was 57.4 (±13.6) years, mean left ventricular ejection fraction was 18.1%, and 66.7% were destination therapy LVADs. No safety events were observed. Successful temporary LVAD weaning was achieved in 50% of MPC and 20% of control patients at 90 days (P=0.24); the posterior probability that MPCs increased the likelihood of successful weaning was 93%. At 90 days, 3 deaths (30%) occurred in control patients, and none occurred in MPC patients. Mean left ventricular ejection fraction after successful wean was 24.0% (MPC=10) and 22.5% (control=2; P=0.56). At 12 months, 30% of MPC patients and 40% of control patients were successfully temporarily weaned from LVAD support (P=0.69), and 6 deaths (30%) occurred in MPC patients. Donor-specific HLA sensitization developed in 2 MPC and 3 control patients and resolved by 12 months.
Conclusions: In this preliminary trial, administration of MPCs appeared to be safe, and there was a potential signal of efficacy. Future studies will evaluate the potential for higher or additional doses to enhance the ability to wean LVAD recipients off support.
Clinical trial registration url: http://www.clinicaltrials.gov. Unique identifier: NCT01442129.
Keywords: heart failure; left ventricular assist device; randomized controlled trial; stem cell.
© 2014 American Heart Association, Inc.
Conflict of interest statement
Figures


References
-
- Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG, Watson JT, Meier P, Ronan NS, Shapiro PA, Lazar RM, Miller LW, Gupta L, Frazier OH, Desvigne-Nickens P, Oz MC, Poirier VL. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–1443. - PubMed
-
- Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM, 3rd, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH Heart Mate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. - PubMed
-
- Russo MJ, Hong KN, Davies RR, Chen JM, Sorabella RA, Ascheim DD, Williams MR, Gelijns AC, Stewart AS, Argenziano M, Naka Y. Post transplant survival is not diminished in heart transplant recipients bridged with implantable left ventricular assist devices. J Thorac Cardiovasc Surg. 2009;138:1425–1432. e1–3. - PubMed
-
- Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, Conte JV, Naka Y, Mancini D, Delgado RM, MacGillivray TE, Farrar DJ, Frazier OH Heart Mate II Clinical Investigators. Use of continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. - PubMed
-
- Ogletree-Hughes ML, Stull LB, Sweet WE, Smedira NG, McCarthy PM, Moravec CS. Mechanical unloading restores beta-adrenergic responsiveness and reverses receptor down regulation in the failing human heart. Circulation. 2001;104:881–886. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- HL088955/HL/NHLBI NIH HHS/United States
- HL088939/HL/NHLBI NIH HHS/United States
- UM1 HL088957/HL/NHLBI NIH HHS/United States
- U01 HL088953/HL/NHLBI NIH HHS/United States
- U01 HL088957/HL/NHLBI NIH HHS/United States
- U01 HL088951/HL/NHLBI NIH HHS/United States
- HL088951/HL/NHLBI NIH HHS/United States
- UM1 HL087318/HL/NHLBI NIH HHS/United States
- UM1 HL088939/HL/NHLBI NIH HHS/United States
- HL088957/HL/NHLBI NIH HHS/United States
- UM1 HL088953/HL/NHLBI NIH HHS/United States
- HL077096/HL/NHLBI NIH HHS/United States
- U01 HL088942/HL/NHLBI NIH HHS/United States
- U01 HL088955/HL/NHLBI NIH HHS/United States
- UM1 HL088955/HL/NHLBI NIH HHS/United States
- HL088953/HL/NHLBI NIH HHS/United States
- U01 HL087366/HL/NHLBI NIH HHS/United States
- UM1 HL087394/HL/NHLBI NIH HHS/United States
- P50 HL077096/HL/NHLBI NIH HHS/United States
- UM1 HL087366/HL/NHLBI NIH HHS/United States
- U01 HL088939/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

