Distinct Z-DNA binding mode of a PKR-like protein kinase containing a Z-DNA binding domain (PKZ)
- PMID: 24682817
- PMCID: PMC4027156
- DOI: 10.1093/nar/gku189
Distinct Z-DNA binding mode of a PKR-like protein kinase containing a Z-DNA binding domain (PKZ)
Abstract
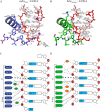
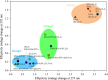
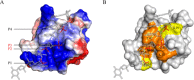
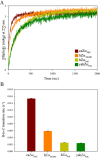
Double-stranded ribonucleic acid-activated protein kinase (PKR) downregulates translation as a defense mechanism against viral infection. In fish species, PKZ, a PKR-like protein kinase containing left-handed deoxyribonucleic acid (Z-DNA) binding domains, performs a similar role in the antiviral response. To understand the role of PKZ in Z-DNA recognition and innate immune response, we performed structural and functional studies of the Z-DNA binding domain (Zα) of PKZ from Carassius auratus (caZαPKZ). The 1.7-Å resolution crystal structure of caZαPKZ:Z-DNA revealed that caZαPKZ shares the overall fold with other Zα, but has discrete structural features that differentiate its DNA binding mode from others. Functional analyses of caZαPKZ and its mutants revealed that caZαPKZ mediates the fastest B-to-Z transition of DNA among Zα, and the minimal interaction for Z-DNA recognition is mediated by three backbone phosphates and six residues of caZαPKZ. Structure-based mutagenesis and B-to-Z transition assays confirmed that Lys56 located in the β-wing contributes to its fast B-to-Z transition kinetics. Investigation of the DNA binding kinetics of caZαPKZ further revealed that the B-to-Z transition rate is positively correlated with the association rate constant. Taking these results together, we conclude that the positive charge in the β-wing largely affects fast B-to-Z transition activity by enhancing the DNA binding rate.
© The Author(s) 2014. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
-
- Holcik M., Sonenberg N. Translational control in stress and apoptosis. Nat. Rev. Mol. Cell Biol. 2005;6:318–327. - PubMed
-
- Samuel C.E. The eIF-2 alpha protein kinases, regulators of translation in eukaryotes from yeasts to humans. J. Biol. Chem. 1993;268:7603–7606. - PubMed
-
- Wek R.C., Jiang H.Y., Anthony T.G. Coping with stress: eIF2 kinases and translational control. Biochem. Soc. Trans. 2006;34:7–11. - PubMed
-
- Williams B.R. PKR: a sentinel kinase for cellular stress. Oncogene. 1999;18:6112–6120. - PubMed
-
- Wu S., Kaufman R.J. A model for the double-stranded RNA (dsRNA)-dependent dimerization and activation of the dsRNA-activated protein kinase PKR. J. Biol. Chem. 1997;272:1291–1296. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

