MS disease activity in RESTORE: a randomized 24-week natalizumab treatment interruption study
- PMID: 24682966
- PMCID: PMC4011468
- DOI: 10.1212/WNL.0000000000000355
MS disease activity in RESTORE: a randomized 24-week natalizumab treatment interruption study
Erratum in
- Neurology. 2015 Feb 24;84(8):862. multiple investigator names added
Abstract
Objective: RESTORE was a randomized, partially placebo-controlled exploratory study evaluating multiple sclerosis (MS) disease activity during a 24-week interruption of natalizumab.
Methods: Eligible patients were relapse-free through the prior year on natalizumab and had no gadolinium-enhancing lesions on screening brain MRI. Patients were randomized 1:1:2 to continue natalizumab, to switch to placebo, or to receive alternative immunomodulatory therapy (other therapies: IM interferon β-1a [IM IFN-β-1a], glatiramer acetate [GA], or methylprednisolone [MP]). During the 24-week randomized treatment period, patients underwent clinical and MRI assessments every 4 weeks.
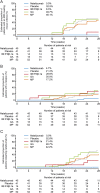
Results: Patients (n = 175) were randomized to natalizumab (n = 45), placebo (n = 42), or other therapies (n = 88: IM IFN-β-1a, n = 17; GA, n = 17; MP, n = 54). Of 167 patients evaluable for efficacy, 49 (29%) had MRI disease activity recurrence: 0/45 (0%) natalizumab, 19/41 (46%) placebo, 1/14 (7%) IM IFN-β-1a, 8/15 (53%) GA, and 21/52 (40%) MP. Relapse occurred in 4% of natalizumab patients and in 15%-29% of patients in the other treatment arms. MRI disease activity recurred starting at 12 weeks (n = 3 at week 12) while relapses were reported as early as 4-8 weeks (n = 2 in weeks 4-8) after the last natalizumab dose. Overall, 50/167 patients (30%), all in placebo or other-therapies groups, restarted natalizumab early because of disease activity.
Conclusions: MRI and clinical disease activity recurred in some patients during natalizumab interruption, despite use of other therapies.
Classification of evidence: This study provides Class II evidence that for patients with MS taking natalizumab who are relapse-free for 1 year, stopping natalizumab increases the risk of MS relapse or MRI disease activity as compared with continuing natalizumab.
Figures

Comment in
-
Natalizumab discontinuation in the increasing complexity of multiple sclerosis therapy.Neurology. 2014 Apr 29;82(17):1484-5. doi: 10.1212/WNL.0000000000000369. Epub 2014 Mar 28. Neurology. 2014. PMID: 24682968 No abstract available.
-
MS disease activity in RESTORE: a randomized 24-week natalizumab treatment interruption study.Neurology. 2014 Nov 25;83(22):2099-100. doi: 10.1212/01.wnl.0000457455.74798.9a. Neurology. 2014. PMID: 25422402 No abstract available.
-
Author response.Neurology. 2014 Nov 25;83(22):2100. Neurology. 2014. PMID: 25574542 No abstract available.
References
-
- Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006;354:899–910 - PubMed
-
- Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 2006;354:911–923 - PubMed
-
- Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 2012;366:1870–1880 - PubMed
-
- Fox RJ, Rudick RA. Risk stratification and patient counseling for natalizumab in multiple sclerosis. Neurology 2012;78:436–437 - PubMed
-
- Major EO. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu Rev Med 2010;61:35–47 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
