Darbepoetin for the anaemia of chronic kidney disease
- PMID: 24683046
- PMCID: PMC10656599
- DOI: 10.1002/14651858.CD009297.pub2
Darbepoetin for the anaemia of chronic kidney disease
Abstract
Background: Erythropoiesis-stimulating agents are used to treat anaemia in people with chronic kidney disease (CKD). Several agents are available including epoetin alfa or beta as well as agents with a longer duration of action, darbepoetin alfa and methoxy polyethylene glycol-epoetin beta.
Objectives: To assess the benefits and harms of darbepoetin alfa to treat anaemia in adults and children with CKD (stages 3 to 5, 5D, and kidney transplant recipients).
Search methods: We searched the Cochrane Renal Group's Specialised Register (to 13 January 2014) through contact with the Trials' Search Co-ordinator using search terms relevant to this review. Studies contained in the Specialised Register are identified through search strategies specifically designed for CENTRAL, MEDLINE and EMBASE.
Selection criteria: We included randomised controlled trials of any darbepoetin alfa treatment of at least three months duration in adults or children with CKD (any stage).
Data collection and analysis: Data were extracted by two independent investigators. Patient-centred outcomes (need for blood transfusion, iron therapy, progression of kidney disease, total and cardiovascular mortality, cardiovascular events, cancer, hypertension, seizures, and health-related quality of life) and other outcomes (haemoglobin levels) were assessed using random effects meta-analysis. We calculated risk ratios for dichotomous outcomes and mean differences for continuous outcomes, both with 95% confidence intervals.
Main results: We identified 32 studies comprising 9414 participants; 21 studies in 8328 participants could be included in our meta-analyses. One study (4038 participants) compared darbepoetin alfa to placebo, 16 studies (2955 participants) compared darbepoetin alfa to epoetin alfa or beta, four studies (1198 participants) compared darbepoetin alfa to methoxy polyethylene glycol-epoetin beta, three studies (420 participants) compared more frequent with less frequent darbepoetin alfa administration and four studies (303 participants) compared intravenous with subcutaneous darbepoetin alfa administration.In a single large study, darbepoetin alfa reduced the need for blood transfusion and iron therapy compared with placebo in adults with CKD stage 3 to 5, but had little or no effect on survival, increased risks of hypertension, and had uncertain effects on quality of life. Data comparing darbepoetin alfa with epoetin alfa or beta or methoxy polyethylene glycol-epoetin beta were sparse and inconclusive. Comparisons of differing dosing schedules and routes of administration were compared in small numbers of participants and studies. Evidence for treatment effects of darbepoetin alfa were particularly limited for children with CKD, adults with CKD stage 5D, and recipients of a kidney transplant.Studies included in this review were generally at high or unclear risk of bias for all items (random sequence generation, allocation concealment, incomplete outcome data, blinding of participants and personnel, blinding of outcome assessment, selective outcome reporting, intention to treat analysis and other sources of bias). One large study comparing darbepoetin alfa with placebo was at low risk of bias for most items assessed.
Authors' conclusions: Data suggest that darbepoetin alfa effectively reduces need for blood transfusions in adults with CKD stage 3 to 5, but has little or no effect on mortality or quality of life. The effects of darbepoetin alfa in adults with CKD stage 5D and kidney transplant recipients and children with CKD remain uncertain as do the relative benefits and harms of darbepoetin alfa compared with other ESAs (epoetin alfa or beta and methoxy polyethylene glycol-epoetin beta).
Conflict of interest statement
Jonathan Craig: None known
Sankar Navaneethan: None known
Suetonia Palmer received a fellowship administered by the Consorzio Mario Negri Sud from Amgen Dompe for assistance with travel for collaboration and supervision. SCP is a L'Oreal For Women in Science Australia and New Zealand Fellow in 2012.
Giovanni Strippoli: None known
Figures
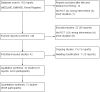


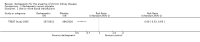






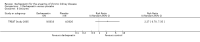








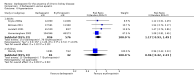
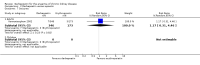



















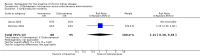



Update of
References
References to studies included in this review
Aarup 2004 {published data only}
-
- Aarup M, Bryndum J, Dieperink H, Joffe P. Clinical implications of converting stable haemodialysis patients from subcutaneous to intravenous administration of darbepoetin alfa. Nephrology Dialysis Transplantation 2006;21(5):1312‐6. [MEDLINE: ] - PubMed
-
- Aarup M, Bryndum J, Joffe P, Dieperink H. Clinical implications of converting stable haemodialysis patients from subcutaneous to intravenous administration of Aranesp® (darbepoetin alfa) [abstract no: PUB278]. Journal of the American Society of Nephrology 2004;15(Oct):821A. [CENTRAL: CN‐00583657] - PubMed
Akizawa 2011 {published data only}
-
- Akizawa T, Gejyo F, Nishi S, Iino Y, Watanabe Y, Suzuki M, et al. Positive outcomes of high hemoglobin target in patients with chronic kidney disease not on dialysis: a randomized controlled study. Therapeutic Apheresis & Dialysis 2011;15(5):431‐40. [MEDLINE: ] - PubMed
-
- Akizawa T, Tsubakihara Y. Target level for hemoglobin correction by darbepoetin alfa (KRN321) for patients with chronic kidney disease (CKD) not on dialysis in randomized controlled study; from the viewpoint of the efficacy [abstract no: SU‐PO804]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):762A. [CENTRAL: CN‐00740554]
-
- Tsubakihara Y, Akizawa T. High target hemoglobin with erythropoiesis stimulating agent (ESA) in chronic kidney disease (CKD) slows the occurrence rate of events related to decline of renal function [abstract no: SA‐FC341]. Journal of the American Society of Nephrology 2009;20:79A. [CENTRAL: CN‐00793981]
-
- Tsubakihara Y, Akizawa T. Target level for hemoglobin correction by darbepoetin alfa (KRN321) for patients with chronic kidney disease (CKD) not on dialysis in randomized controlled study; from the viewpoint of the safety [abstract no: SU‐PO818]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):765A. [CENTRAL: CN‐00740542]
Allon 2002 {published data only}
-
- Allon M, Kleinman AK, Walczyk M, Kaupke C, Maroni BJ, Heatherington A, et al. The pharmacokinetics of novel erythropoiesis stimulating protein (NESP) following chronic intravenous administration is time‐and dose‐linear [abstract no: A1308]. Journal of the American Society of Nephrology 2000;11(Sept):248A. [CENTRAL: CN‐00626053]
-
- Allon M, Kleinman K, Walczyk M, Kaupke C, Messer‐Mann L, Olson K, et al. Pharmacokinetics and pharmacodynamics of darbepoetin alfa and epoetin in patients undergoing dialysis. Clinical Pharmacology & Therapeutics 2002;72(5):546‐55. [MEDLINE: ] - PubMed
ARCTOS Study 2008 {published data only}
-
- Fishbane S, Dalton C, Beswick R, Dutka P, Schmidt R. Efficacy of C.E.R.A., a continuous erythropoietin receptor activator, in the treatment of renal anemia: overview of 6 global phase 3 trials [abstract no: 59]. American Journal of Kidney Diseases 2007;49(4):A39. [CENTRAL: CN‐00756571]
-
- Kessler M, Martinez‐Castelao A, Siamopoulos KC, Villa G, Spinowitz B, Dougherty FC, et al. C.E.R.A. once every 4 weeks in patients with chronic kidney disease not on dialysis: The ARCTOS extension study. Hemodialysis International 2010;14(2):233‐9. [MEDLINE: ] - PubMed
-
- Macdougall IC, Walker R, Provenzano R, Alvaro F, Locay HR, Nader PC, et al. C.E.R.A. corrects anemia in patients with chronic kidney disease not on dialysis: results of a randomized clinical trial. Clinical Journal of The American Society of Nephrology: CJASN 2008;3(2):337‐47. [MEDLINE: ] - PMC - PubMed
-
- Macdougall IC, Walker R, Provenzano R, Alvaro F, Locay HR, Nader PC, et al. C.E.R.A. (Continuous Erythropoietin Receptor Activator) administered at extended intervals corrects anemia and maintains stable Hb levels in patients with CKD not on dialysis [abstract no: SA‐PO208]. Journal of the American Society of Nephrology 2006;17(Abstracts):619A. [CENTRAL: CN‐00644214]
-
- Provenzano R, Macdougall IC, Law A, Ouyang Y, Bexon M. Anemia correction with C.E.R.A. in patients (pts) with chronic kidney disease (CKD) is unaffected by baseline hemoglobin (Hb) level [abstract no: SU‐PO796]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):760A. [CENTRAL: CN‐00747311]
Bommer 2004 {published data only}
-
- Bommer J, Asmus G, Wenning M, Bommer G. A comparison of haemoglobin levels and doses in haemodialysis patients treated with subcutaneous or intravenous darbepoetin alfa: a German prospective, randomized, multicentre study. Nephrology Dialysis Transplantation 2008;23(12):4002‐8. [MEDLINE: ] - PubMed
-
- Bommer J, Asmus H, Bommer G, Wenning M. Efficacy of IV or SC darbepoetin alfa in hemodialysis patients [abstract no: W‐PO40135]. Nephrology 2005;10(Suppl 1):A315. [CENTRAL: CN‐00602087]
-
- Bommer J, Assmus H, Bommer G, Wenning M. Comparison of IV or SC darbepoetin alfa in hemodialysis patients [abstract no: F‐PO499]. Journal of the American Society of Nephrology 2004;15(Oct):176A. [CENTRAL: CN‐00583725]
-
- Bommer J, Wenning M, Bommer G. Comparison of IV or SC darbepoetin in hemodialysis patients [abstract no: F‐PO676]. Journal of the American Society of Nephrology 2005;16:482A. [CENTRAL: CN‐00583778]
Cervelli 2005 {published data only}
-
- Cervelli MJ, Disney APS, Gray N, McDonald SP. Randomised, single blinded, cross‐over comparison of intravenous and subcutaneous darbepoetin dosing efficiency in haemodialysis patients [abstract]. Nephrology 2004;9(Suppl 1):A28. [CENTRAL: CN‐00509125] - PubMed
-
- Cervelli MJ, Gray N, McDonald S, Gentgall MG, Disney AP. Randomized cross‐over comparison of intravenous and subcutaneous darbepoetin dosing efficiency in haemodialysis patients. Nephrology 2005;10(2):129‐35. [MEDLINE: ] - PubMed
Chazot 2009 {published data only}
-
- Chazot C, Dumoulin A, Ang KS, Paul GJ, Bernard C, Conference Medicale Group. Haemodialysis patients under subcutaneous recombinant human erythropoietin can be directly switched to intravenous Aranesp® [abstract no: F‐PO682]. Journal of the American Society of Nephrology 2005;16:483A. [CENTRAL: CN‐00740583]
-
- Chazot C, Terrat JC, Ang K, Gassia JP, Chedid K, Maurice F, et al. Intravenous darbepoetin A maintains hemoglobin level in hemodialysis patients converting from subcutaneous recombinant human erythropoietin. [abstract no: F‐PO501]. Journal of the American Society of Nephrology 2004;15(Oct):177A. [CENTRAL: CN‐00583168]
-
- Chazot C, Terrat JC, Dumoulin A, Ang KS, Gassia JP, Chedid K, et al. Randomized equivalence study evaluating the possibility of switching hemodialysis patients receiving subcutaneous human erythropoietin directly to intravenous darbepoetin alfa. Annals of Pharmacotherapy 2009;43(2):228‐34. [MEDLINE: ] - PubMed
Coyne 2000 {published data only}
-
- Coyne DW, Ling BN, Toto R, McDermott‐Vitak AD, Trotman ML, Jackson L. Novel erythropoiesis stimulating protein (NESP) corrects anemia in dialysis patients when administered at reduced dose frequency compared with recombinant‐human erythropoietin (R‐HUEPO) [abstract no:1380]. Journal of the American Society of Nephrology 2000;11(Sept):263A. [CENTRAL: CN‐00583382]
Coyne 2006a {published data only}
-
- Coyne D, Zeig R, Benz R, Berns J, Varma N, Nakanishi A, et al. A randomized, double‐blind study comparing darbepoetin alfa and recombinant human erythropoietin (rHuEPO) in the treatment of anemia in African‐American (AA) subjects with chronic kidney disease (CKD) receiving hemodialysis (HD) [abstract no:TH‐PO365]. Journal of the American Society of Nephrology 2006;17(Abstracts):184A. [CENTRAL: CN‐00740574]
-
- NCT00111995. A randomized, double‐blind study comparing Aranesp© (darbepoetin alfa) and recombinant human erythropoietin (rHuEPO) in the treatment of anemia in African‐American subjects with chronic renal failure (CRF) receiving hemodialysis. www.clinicaltrials.gov/ct2/show/NCT00111995 (accessed 16 January 2014).
Hirakata 2010 {published data only}
-
- Hirakata H, Gejyo F, Suzuki M, Saito A, Lino Y, Watanabe Y, et al. Effect of darbepoetin alfa (KRN321) subcutaneous treatment on hemoglobin levels, health‐related QOL (HRQOL) and left ventricular mass index (LVMI) in patients with chronic kidney disease (CKD) not on dialysis [abstract no: SA‐PO204]. Journal of the American Society of Nephrology 2006;17(Abstracts):618A. [CENTRAL: CN‐00740524]
-
- Hirakata H, Tsubakihara Y, Gejyo F, Nishi S, Iino Y, Watanabe Y, et al. Maintaining high hemoglobin levels improved the left ventricular mass index and quality of life scores in pre‐dialysis Japanese chronic kidney disease patients. Clinical & Experimental Nephrology 2010;14(1):28‐35. [MEDLINE: ] - PubMed
-
- Inaguma D, Tsubakihara Y, Hirakata H, Hiroe M, Hada Y, Akizawa T, et al. Monthly subcutaneous treatment of darbepoetin alfa (KRN321) could maintain higher Hb safely and have beneficial effects on cardiac function of Japanese CKD patients not on dialysis [abstract no: SaP353]. Nephrology Dialysis Transplantation 2007;22(Suppl 6):vi352. [CENTRAL: CN‐00740573]
-
- Suzuki M, Hada Y, Akaishi M, Hiroe M, Aonuma K, Tsubakihara Y, et al. Effects of anemia correction by erythropoiesis‐stimulating agents on cardiovascular function in non‐dialysis patients with chronic kidney disease. International Heart Journal 2012;53(4):238‐43. [MEDLINE: ] - PubMed
Hori 2004 {published data only}
-
- Hori K, Tsujimoto Y, Ohmori H, Nakamura H, Suga A, Iwasaki M, et al. Randomized, double‐blind, comparative study of intravenous KRN321 (darbepoetin alfa) compared to intravenous recombinant human erythropoietin (rHuEPO) for treatment of anemia in subjects with chronic renal failure (CRF) receiving hemodialysis in Japan [abstract no:F‐PO502]. Journal of the American Society of Nephrology 2004;15(Oct):177A. [CENTRAL: CN‐00740529]
Iwasaki 2008 {published data only}
-
- Iwasaki T, Kimata N, Sugi O, Sawara Y, Jinnai H, Kikuchi K, et al. Difference in reactivity became more evident by changing of the erythropoiesis‐stimulating agent (ESA) [abstract no: PUB462]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):914A. [CENTRAL: CN‐00792600]
Kim 2009a {published data only}
-
- Kim CD, Park SH, Kim DJ, Do JY, Shin SK, Kim BS, et al. Randomized trial to compare the dosage of darbepoetin alfa by administration route in hemodialysis patient [abstract no: TH‐PO370]. Journal of the American Society of Nephrology 2006;17(Abstracts):185A. [CENTRAL: CN‐00740537] - PubMed
-
- Kim CD, Park SH, Kim DJ, Park JW, Do JY, Shin SK, et al. Randomized trial to compare the dosage of darbepoetin alfa by administration route in haemodialysis patients. Nephrology 2009;14(5):482‐7. [MEDLINE: ] - PubMed
Kwan 2005 {published data only}
-
- Kwan BC, Leung C, Szeto C, Li PK. Efficacy of bi‐weekly versus monthly subcutaneous darbepoetin‐alpha for the maintenance treatment of anemia in peritoneal dialysis patients ‐ an open‐label randomized study [abstract no:SA‐PO805]. Journal of the American Society of Nephrology 2005;16:732A. [CENTRAL: CN‐00740570]
Li 2008 {published data only}
-
- Li WY, Chu TS, Huang JW, Wu MS, Wu KD. Randomized study of darbepoetin alfa and recombinant human erythropoietin for treatment of renal anemia in chronic renal failure patients receiving peritoneal dialysis. Journal of the Formosan Medical Association 2008;107(11):843‐50. [MEDLINE: ] - PubMed
Locatelli 2001 {published data only}
-
- Johnson DW, European/Australian NESP 980202 Study Group. Novel erythropoiesis stimulating protein (darbepoetin alpha) corrects anaemia of early chronic kidney disease (CKD) at a reduced dose frequency compared with recombinant human erythropoietin (rHuEPO) [abstract no: P156]. Nephrology 2002;7(Suppl 3):A40. [CENTRAL: CN‐00794721]
-
- Locatelli F, European/Australian NESP 980202 Study Group. Novel erythropoiesis stimulating protein (NESP) corrects anemia of chronic renal insufficiency (CRI) at a reduced dose frequency compared with rHuEPO [abstract]. Nephrology Dialysis Transplantation 2001;16(6):A92. [CENTRAL: CN‐00671771]
-
- Locatelli F, Olivares J, Walker R, Wilkie M, European/Australian NESP 980202 Study Group. Novel erythropoiesis stimulating protein (NESP) administered subcutaneously corrects anemia in subjects with chronic renal insufficiency (CRI) when administered at a reduced dose frequency compared with recombinant‐human erythropoietin (r‐HuEPO) [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):283A. [CENTRAL: CN‐00626023]
-
- Locatelli F, Olivares J, Walker R, Wilkie M, Jenkins B, Dewey C, et al. Novel erythropoiesis stimulating protein for treatment of anemia in chronic renal insufficiency. Kidney International 2001;60(2):741‐7. [MEDLINE: ] - PubMed
Locatelli 2004 {published data only}
-
- Locatelli F, Backs W, Pino MD, Martin A, Guillaume B, Vikydal R, et al. Control of anaemia with Aranesp®: haemoglobin stability achieved with fewer patients requiring dose adjustments after switching to Aranesp® every 2‐weeks [abstract no: SP411]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv152. [CENTRAL: CN‐00740593]
-
- Locatelli F, Villa G, Backs W, Pino MD. A phase 3, multicentre, randomised, double‐blind, non‐inferiority trial to evaluate the efficacy of Aranesp® (darbepoetin alfa) once every 2 weeks (Q2W) vs Aranesp® once weekly (QW) inpatients on haemodialysis (HD) [abstract no: MP181]. Nephrology Dialysis Transplantation 2005;20(Suppl 5):v260. [CENTRAL: CN‐00740588]
-
- Locatelli F, Villa G, Backs W, Pino MD. Aranesp® (darbepoetin alfa) maintains hemoglobin (Hb) levels in hemodialysis (HD) patients at extended dosing intervals regardless of previous recombinant human erythropoietin (rHuEPO) dosing frequency [abstract no: PUB254]. Journal of the American Society of Nephrology 2004;15(Oct):816A. [CENTRAL: CN‐00740589]
Locatelli 2008 {published data only}
-
- Locatelli F, Villa G, Messa P, Filippini A, Cannella G, Ferrari G, et al. Efficacy and safety of once‐weekly intravenous epoetin alfa in maintaining hemoglobin levels in hemodialysis patients. Journal of Nephrology 2008;21(3):412‐20. [MEDLINE: ] - PubMed
Nagaya 2010 {published data only}
-
- Inaguma D, Nagaya H, Hamaguchi K, Tatematsu M, Suzuki S, Kurata K. Intravenous darbepoetin alfa once a week could maintain hemoglobin level more efficiently than once every 2 weeks in Japanese patients on hemodialysis [abstract no: TH‐PO741]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):275A. [CENTRAL: CN‐00740572] - PubMed
-
- Nagaya H, Inaguma D, Kitagawa A, Murata M, Kamimura Y, Hamaguchi K, et al. Intravenously administered darbepoetin alfa once a week could maintain hemoglobin level more efficiently than once every 2 weeks in patients on hemodialysis. Clinical & Experimental Nephrology 2010;14(2):158‐63. [MEDLINE: ] - PubMed
Nissenson 2002 {published data only}
-
- Nissenson A, Krishnan M, Liu W, McCary L, Stehman‐Breen C, Mix C. Hemoglobin (Hb) variability does not differ between hemodialysis (HD) patients treated with epoetin alfa and darbepoetin alfa [abstract no: TH‐PO360]. Journal of the American Society of Nephrology 2006;17(Abstracts):183A. [CENTRAL: CN‐00740539]
-
- Nissenson AR. Dosing darbepoetin alfa. American Journal of Kidney Diseases 2002;40(4):872. [MEDLINE: ] - PubMed
-
- Nissenson AR, Swan SK, Lindberg JS, Soroka SD, Beatey R, Wang C, et al. Randomized, controlled trial of darbepoetin alfa for the treatment of anemia in hemodialysis patients. American Journal of Kidney Diseases 2002;40(1):110‐8. [MEDLINE: ] - PubMed
-
- Nissenson AR, Swan SK, Lindberg JS, Soroka SD, McDermott‐Vitak AD, Wang C, et al. Novel Erythropoiesis stimulating protein (NESP) safely maintains hemoglobin concentration levels in hemodialysis patients as effectively as r‐HuEPO when administered once weekly [abstract no: A1326]. Journal of the American Society of Nephrology 2000;11(Sept):252A. [CENTRAL: CN‐00794722]
-
- Nissenson AR, US/Canadian NESP 980117 Study Group. Once weekly IV novel erythropoiesis stimulating protein (NESP) effectively maintains Hb in hemodialysis patients [abstract]. Nephrology Dialysis Transplantation 2001;16(6):A92. [CENTRAL: CN‐00446969]
PATRONUS Study 2010 {published data only}
-
- Carrera F. CERA vs darbepoetin alfa as maintenance therapy for anaemia in patients with chronic kidney disease (CKD): The PATRONUS Study [abstract no: M558]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009.
-
- Carrera F, Lok CE, Francisco A, Locatelli F, Mann JF, Canaud B, et al. Maintenance treatment of renal anaemia in haemodialysis patients with methoxy polyethylene glycol‐epoetin beta versus darbepoetin alfa administered monthly: a randomized comparative trial. Nephrology Dialysis Transplantation 2010;25(12):4009‐17. [MEDLINE: ] - PMC - PubMed
-
- Locatelli F, PATRONUS Study Group. Once‐monthly C.E.R.A. is superior to darbepoetin alfa for maintaining hemoglobin levels in hemodialysis patients regardless of age, gender, diabetic status or presence of hyperlipidemia [abstract no: SA‐PO2412]. Journal of the American Society of Nephrology 2009;20:663A. [CENTRAL: CN‐00747328]
-
- Mann JF, PATRONUS Study Group. Risk factors for vascular events in hemodialysis patients receiving once‐monthly C.E.R.A. or once‐monthly darbepoetin alfa: post hoc analysis of the PATRONUS Study [abstract no: PUB414]. Journal of the American Society of Nephrology 2009;20:921A. [CENTRAL: CN‐00747329]
-
- Francisco AL, PATRONUS Study Group. Significantly higher hemoglobin response rates are achieved in hemodialysis patients with once‐monthly C.E.R.A. compared with darbepoetin alfa in hemodialysis, regardless of etiology of chronic kidney disease [abstract no: SA‐PO2411]. Journal of the American Society of Nephrology 2009;20:663A. [CENTRAL: CN‐00747327]
Smyth 2004 {published data only}
-
- Smyth JS, Marshall W, Ashfield L, Elias L, McGinn S. Conversion from subcutaneous to intravenous erythropoietin analogues is cost neutral: results of a six month pilot study [abstract no: PUB246]. Journal of the American Society of Nephrology 2004;15(Oct):814A‐5A. [CENTRAL: CN‐00792440]
STRIATA Study 2008 {published data only}
-
- Barany P, Besarab A, Macdougall IC, Law A, Ouyang Y, Heifets M. Median hemoglobin (Hb) decline following C.E.R.A. dose interruption is similar to that with other erythropoiesis stimulating agents (ESAs) [abstract no: SU‐PO795]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):760A. [CENTRAL: CN‐00794522]
-
- Canaud B, Braun J, Locatelli F, Villa G, Vlem B, Sanz Guajardo D, et al. Intravenous (IV) C.E.R.A. (continuous erythropoietin receptor activator) administered once every 2 weeks maintains stable haemoglobin (Hb) levels in patients with chronic kidney disease (CKD) on dialysis [abstract no: SP425]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv157. [CENTRAL: CN‐00690645]
-
- Canaud B, Mingardi G, Braun J, Aljama P, Kerr PG, Locatelli F, et al. Intravenous C.E.R.A. maintains stable haemoglobin levels in patients on dialysis previously treated with darbepoetin alfa: results from STRIATA, a randomized phase III study. Nephrology Dialysis Transplantation 2008;23(11):3654‐61. [MEDLINE: ] - PMC - PubMed
-
- Fishbane S, Dalton C, Beswick R, Dutka P, Schmidt R. Efficacy of C.E.R.A., a continuous erythropoietin receptor activator, in the treatment of renal anemia: overview of 6 global phase 3 trials [abstract no: 59]. American Journal of Kidney Diseases 2007;49(4):A39. [CENTRAL: CN‐00756571]
Svarstad 2007 {published data only}
-
- Svarstad E, Forslund T. Nurse assisted nine months darbepoetin (D) treatment with fix dose and variable dosing interval in an outpatient clinic is cost effective in predialytic CKD patients [abstract no: SU‐PO800]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):761A. [CENTRAL: CN‐00740562]
Tessitore 2008 {published data only}
-
- Tessitore N, Mantovani W, Bedogna V, Loss R, Melilli E, Poli A, et al. Cost analysis of switching from epoetin alfa to darbepoetin alfa in chronic hemodialysis patients (HD Pts): a long‐term, randomized, open‐label, cross‐over, pilot study [abstract no: SA‐PO2645]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):706A. [CENTRAL: CN‐00740545]
TIVOLI Study 2011 {published data only}
-
- Campistol JM, Carreno A, Morales JM, Pallardo L, Franco A, Navarro D, et al. Once‐monthly pegylated epoetin Beta versus darbepoetin alfa every two weeks in renal transplant recipients: a randomized trial. Transplantation 2013;95(2):e6‐e10. [MEDLINE: ] - PubMed
-
- Carreno A, Campistol JM, Arias M, Morales JM, Pallardo L, Franco A. A randomised, multicenter, phase IIIb clinical trial to evaluate efficacy and safety of C.E.R.A. once a month versus darbepoetin alfa in renal transplant recipients with chronic renal anaemia (TIVOLI study group) [abstract no: 27]. American Journal of Transplantation 2011;11:36. [EMBASE: 70405062]
-
- Carreno A, Campistol JM, Arias M, Morales JM, Pallardo L, Franco A. A randomised, multicenter, phase IIIb clinical trial to evaluate efficacy and safety of CERA once a month versus darbepoetin alfa in renal transplant recipients with chronic renal anaemia (TIVOLI Study Group) [abstract no: F164]. NDT Plus 2011;4(Suppl 2):4.
Tolman 2005 {published data only}
-
- Tolman C, Richardson D, Bartlett C, Will E. A randomised study of weekly subcutaneous Aranesp and Neorecormon in a large unselected haemodialysis cohort, managed with computer assisted anaemia algorithms [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:229‐30. [CENTRAL: CN‐00509514]
-
- Tolman C, Richardson D, Bartlett C, Will E. Application of computer assisted anaemia management algorithms in haemodialysis patients produces predictable haemoglobin outcomes regardless of the erythropoietic agent or frequency of administration: results of a randomised study [abstract]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:110. [CENTRAL: CN‐00509513]
-
- Tolman C, Richardson D, Bartlett C, Will E. Structured conversion from thrice weekly to weekly erythropoietic regimens using a computerized decision‐support system: a randomized clinical study. Journal of the American Society of Nephrology 2005;16(5):1463‐70. [MEDLINE: ] - PubMed
-
- Tolman C, Richardson D, Bartlett C, Will EJ. Dose conversion ratio (DCR) from subcutaneous epoetin‐B (EPO) to weekly darbepoetin‐A (DA) is dependent on baseline sensitivity: results from a randomised study [abstract no: SA‐PO461]. Journal of the American Society of Nephrology 2004;15(Oct):404A. [CENTRAL: CN‐00583282]
-
- West RM, Harris K, Gilthorpe MS, Tolman C, Will EJ. Functional data analysis applied to a randomized controlled clinical trial in hemodialysis patients describes the variability of patient responses in the control of renal anemia. Journal of the American Society of Nephrology 2007;18(8):2371‐6. [MEDLINE: ] - PubMed
TREAT Study 2005 {published data only}
-
- Desai AS, Toto R, Jarolim P, Uno H, Eckardt KU, Kewalramani R, et al. Association between cardiac biomarkers and the development of ESRD in patients with type 2 diabetes mellitus, anemia, and CKD. American Journal of Kidney Diseases 2011;58(5):717‐28. [MEDLINE: ] - PubMed
-
- McMurray JJ, Uno H, Jarolim P, Desai AS, Zeeuw D, Eckardt KU, et al. Predictors of fatal and nonfatal cardiovascular events in patients with type 2 diabetes mellitus, chronic kidney disease, and anemia: an analysis of the Trial to Reduce cardiovascular Events with Aranesp (darbepoetin‐alfa) Therapy (TREAT). American Heart Journal 2011;162(4):748‐55. [MEDLINE: ] - PubMed
-
- Mix TC, Brenner RM, Cooper ME, Zeeuw D, Ivanovich P, Levey AS, et al. Rationale‐‐Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT): evolving the management of cardiovascular risk in patients with chronic kidney disease. American Heart Journal 2005;149(3):408‐13. [MEDLINE: ] - PubMed
-
- Pfeffer MA, Burdmann EA, Chen C, Cooper ME, Zeeuw D, Eckardt K, et al. Trial to reduce cardiovascular events with Aranesp Therapy [abstract]. Circulation 2010;120(21):2154‐5. [EMBASE: 70089295]
Vanrenterghem 2002 {published data only}
-
- Canaud B. Darbepoetin alfa dose requirements for IV and SC administration are equivalent in anaemic dialysis patients [abstract no: M319]. Nephrology Dialysis Transplantation 2002;17(Suppl 1):137. [CENTRAL: CN‐00550478]
-
- Kerr PG, Harris D, Hawley C, Walker R, European/Australian NESP 970290 Study Group. Novel erythropoiesis stimulating protein (NESP) maintains haemoglobin in ESRD patients with administered once weekly or once every other week [abstract no: 181]. Nephrology 2000;5(3):A112. [CENTRAL: CN‐00509271]
-
- Vanrenterghem Y, Barany P, Mann J, European/Australian NESP 970290 Study Group. Novel erythropoiesis stimulating protein (NESP) maintains hemoglobin (hgb) in ESRD patients when administered once weekly or once every other week [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):270A. [CENTRAL: CN‐00583820]
-
- Vanrenterghem Y, Barany P, Mann JF, Kerr PG, Wilson J, Baker NF, et al. Randomized trial of darbepoetin alfa for treatment of renal anemia at a reduced dose frequency compared with rHuEPO in dialysis patients. Kidney International 2002;62(6):2167‐75. [MEDLINE: ] - PubMed
Warady 2006 {published data only}
-
- Warady BA, Arar MY, Lerner G, Nakanishi AM, Stehman‐Breen C. Darbepoetin alfa for the treatment of anemia in pediatric patients with chronic kidney disease. Pediatric Nephrology 2006;21(8):1144‐52. [MEDLINE: ] - PubMed
Watanabe 2004 {published data only}
-
- Watanabe Y, Gejyo F, Shigematsu T, Iino Y, Hosoya T, Shoji T, et al. KRN321 (darbepoetin alfa) administered once every other week (Q2W) and once every four weeks (Q4W) in predialysis chronic renal failure (CRF) patients in Japan [abstract no: SU‐PO053]. Journal of the American Society of Nephrology 2004;15(Oct):544A. [CENTRAL: CN‐00740541]
Yoon 2004 {published data only}
-
- Yoon SY, Bang BK, Kim SG, Han DC, Hwang SD, Park JS, et al. Randomized, controlled trial of darbepoetin alfa for the treatment of renal anemia in hemodialysis patients [abstract no: MP271]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:322. [CENTRAL: CN‐00509573]
References to studies excluded from this review
Akizawa 2007b {published data only}
-
- Akizawa T, Koshikawa S, Iwasaki M, KRN321 A08 Study Group. Darbepoetin alfa effectively maintains hemoglobin concentrations at extended dose intervals relative to intravenous rHuEPO in Japanese dialysis patients. Therapeutic Apheresis & Dialysis 2007;11(3):220‐6. [MEDLINE: ] - PubMed
Argani 2009 {published data only}
-
- Argani H, Ossareh S, Tabiban S, Ganji MR, Nafar M, Abdi E, et al. PDpoietin in comparison with Eprex in treatment of anemic patients on hemodialysis. Iranian journal of Kidney Diseases 2009;3(3):145‐50. [MEDLINE: ] - PubMed
Carrera 2003 {published data only}
-
- Carrera F, Anunciada AI, Nogueira C, Silva JG. Comparison of HB levels in dialysis patients receiving three‐times weekly rHuepo switched to once‐weekly darbepoetin alfa: results of a randomized study [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):164. [CENTRAL: CN‐00444694]
Chen 2008 {published data only}
-
- Chen HH, Tarng DC, Lee KF, Wu CY, Chen YC. Epoetin alfa and darbepoetin alfa: effects on ventricular hypertrophy in patients with chronic kidney disease. Journal of Nephrology 2008;21(4):543‐9. [MEDLINE: ] - PubMed
COMFORT Study 2007 {published data only}
-
- Roger SD, Suranyi MG, Walker RG, Disney A, Isbel NM, Kairaitis L, et al. A randomised, cross‐over study comparing injection site pain with subcutaneous epoetin beta and subcutaneous darbepoetin alfa in patients with chronic kidney disease. Current Medical Research & Opinion 2008;24(8):2181‐7. [MEDLINE: ] - PubMed
-
- Suranyi M, Walker R, Disney A, Isbel N, Kairaitis L, Pollock C, et al. Subcutaneous neoRecormon (epoetin beta) is associated with less injection site pain than aranesp (darbepoetin alfa) in patients with chronic kidney disease [abstract no: PUB475]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):934A. [CENTRAL: CN‐00740565]
Deray 2003 {published data only}
-
- Deray G. Dosing darbepoetin alfa continued. American Journal of Kidney Diseases 2003;41(6):1334‐5. [MEDLINE: ] - PubMed
Disney 2007 {published data only}
-
- Disney A, Jersey PD, Kirkland G, Mantha M, Charlesworth JA, Gallagher M, et al. Darbepoetin alfa administered monthly maintains haemoglobin concentrations in patients with chronic kidney disease not receiving dialysis: a multicentre, open‐label, Australian study. Nephrology 2007;12(1):95‐101. [MEDLINE: ] - PubMed
Iino 2003 {published data only}
-
- Iino Y, Hiramatsu M, Akizawa T, Koshikawa S, KRN321 Study Group. Subcutaneous (SC) administration of darbepoetin alfa (KRN321) on CAPD patients. Study for pharmacokinetics and safety [abstract no: PUB401]. Journal of the American Society of Nephrology 2003;14(Nov):860A. [CENTRAL: CN‐00740536]
Kawanishi 2005 {published data only}
-
- Kawanishi H, Iwasaki M, Akizawa T, Koshikawa S, KRN321 Study Group. Dose‐finding and long‐term studies of intravenous KRN321 (darbepoetin alfa) in chronic renal failure patients (CRF) on hemodialysis (HD) in Japan [abstract no: SA‐PO943]. Journal of the American Society of Nephrology 2005;16:763A. [CENTRAL: CN‐00740571]
Lamas 2006 {published data only}
-
- Lamas JM, Alonso M, Sastre F, Garcia‐Trio G, Saavedra J, Palomares L. Ultrapure dialysate and inflammatory response in haemodialysis evaluated by darbepoetin requirements‐‐a randomized study. Nephrology Dialysis Transplantation 2006;21(10):2851‐8. [MEDLINE: ] - PubMed
Lerner 2002 {published data only}
-
- Lerner G, Kale AS, Warady BA, Jabs K, Bunchman TE, Heatherington A, et al. Pharmacokinetics of darbepoetin alfa in pediatric patients with chronic kidney disease. Pediatric Nephrology 2002;17(11):933‐7. [MEDLINE: ] - PubMed
Macdougall 1999 {published data only}
-
- Macdougall IC, Gray SJ, Elston O, Breen C, Jenkins B, Browne J, et al. Pharmacokinetics of novel erythropoiesis stimulating protein compared with epoetin alfa in dialysis patients. Journal of the American Society of Nephrology 1999;10(11):2392‐5. [MEDLINE: ] - PubMed
-
- Macdougall IC, Gray SJ, McEvoy O, Breen C, Jenkins B, Browne J, et al. Comparison of the pharmacokinetics of novel erythropoiesis stimulating protein (NESP) and epoetin alfa (rhEPO) in dialysis patients [abstract no: A1233]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):268A. [CENTRAL: CN‐00446519] - PubMed
Macdougall 2003 {published data only}
-
- Macdougall IC. Novel erythropoiesis stimulating protein (NESP) for the treatment of anaemia in ESRD patients [abstract]. 37th Congress. European Renal Association. European Dialysis and Transplantation Association. European Kidney Research Organisation; 2000 Sept 17‐20; Nice, France. 2000:237. [CENTRAL: CN‐00461229]
-
- Macdougall IC. Novel erythropoiesis stimulating protein (NESP) for the treatment of anaemia in ESRD patients [abstract]. Nephrology Dialysis Transplantation 2000;15(9):A160.
-
- Macdougall IC, Matcham J, Gray SJ, NESP 960245/246 Study Group. Correction of anaemia with darbepoetin alfa in patients with chronic kidney disease receiving dialysis. Nephrology Dialysis Transplantation 2003;18(3):576‐81. [MEDLINE: ] - PubMed
-
- Macdougall IC, UK NESP Study Group. Novel erythropoiesis stimulating protein (NESP) for the treatment of renal anaemia [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):258A‐9A. [CENTRAL: CN‐00446518]
Mann 2007 {published data only}
-
- Mann J, Kessler M, Villa G, Martinez‐Castelao A, Feldt‐Rasmussen B, Cruz J, et al. Darbepoetin alfa once every 2 weeks for treatment of anemia in dialysis patients: a combined analysis of eight multicenter trials. Clinical Nephrology 2007;67(3):140‐8. - PubMed
McMahon 2004 {published data only}
-
- McMahon LP, Chester K, Walker RG. Effects of different dialysis membranes on serum concentrations of epoetin alfa, darbepoetin alfa, enoxaparin and iron sucrose during dialysis [abstract no: P109]. Nephrology 2004;9(Suppl 1):A28. [CENTRAL: CN‐00509348] - PubMed
Murtagh 2000 {published data only}
-
- Murtagh H, Brown JH, Maxwell AP. Experience with novel erythropoiesis stimulating protein (NESP) in the treatment of anaemia in haemodialysis patients [abstract]. Irish Journal of Medical Science 2000;169(4):321. [CENTRAL: CN‐00740599]
Paganini 1989 {published data only}
-
- Paganini EP, Latham D, Abdulhadi M. Practical considerations of recombinant human erythropoietin therapy. American Journal of Kidney Diseases 1989;14(2 Suppl 1):19‐25. - PubMed
Schmitt 2006 {published data only}
-
- Schaefer F, Brummer C, Rosenkranz J, Schmitt CP. Increased subcutaneous injection pain with darbepoetin‐A compared with epoetin‐B in children: results of a randomized, double‐blind study [abstract no: P399]. Pediatric Nephrology 2004;19(9):C191. [CENTRAL: CN‐00740567]
-
- Schmitt CP, Brummer C, Rosenkranz J, Schaefer F. Increased injection pain with darbepoetin‐A compared to epoetin‐B: a double‐blind study in pediatric patients [abstract no: SU‐PO068]. Journal of the American Society of Nephrology 2004;15(Oct):547A. [CENTRAL: CN‐00740568]
-
- Schmitt CP, Brummer C, Rosenkranz J, Schaefer F. Injection pain is increased with darbepoetin‐A compared to epoetin‐B [abstract no: W‐PO40120]. Nephrology 2005;10(Suppl 1):A311. [CENTRAL: CN‐00740569]
-
- Schmitt CP, Nau B, Brummer C, Rosenkranz J, Schaefer F. Increased injection pain with darbepoetin‐alpha compared to epoetin‐beta in paediatric dialysis patients. Nephrology Dialysis Transplantation 2006;21(12):3520‐4. [MEDLINE: ] - PubMed
Smith 2007 {published data only}
-
- Pratt R. Pharmacokinetics of erythropoietin by a human cell line (Epoetin I): subcutaneous vs intravenous dosing in patients with chronic kidney disease [abstract no: 1137]. Haematologica 2006;91(Suppl 1):414. [CENTRAL: CN‐00716122]
-
- Pratt RD. Epoetin delta for the treatment of anemia in patients with CKD not requiring hemodialysis [abstract no: TH‐PO378]. Journal of the American Society of Nephrology 2006;17(Abstracts):187A. [CENTRAL: CN‐00765050]
-
- Pratt RD, Dowell J. Pharmacokinetics of epoetin delta: a new erythropoietin produced by gene‐activation in a human cell line [abstract no: TH‐PO377]. Journal of the American Society of Nephrology 2006;17(Abstracts):187A. [CENTRAL: CN‐00644158]
-
- Smith WB, Dowell JA, Pratt RD. Pharmacokinetics and pharmacodynamics of epoetin delta in two studies in healthy volunteers and two studies in patients with chronic kidney disease. Clinical Therapeutics 2007;29(7):1368‐80. [MEDLINE: ] - PubMed
St Peter 1998 {published data only}
-
- Peter WL, Lewis MJ, Macres MG. Pain comparison after subcutaneous administration of single‐dose formulation versus multidose formulation of epogen in hemodialysis patients. American Journal of Kidney Diseases 1998;32(3):470‐4. [MEDLINE: ] - PubMed
Thadhani 2002 {published data only}
-
- Alexander M, Kewalramani R, Agodoa I, Globe D. Association of anemia correction with health related quality of life in patients not on dialysis. Current Medical Research & Opinion 2007;23(12):2997‐3008. [MEDLINE: ] - PubMed
-
- Thadhani R, Cheriyan R, Brenner R, Ford J, Powers K, Rahman SN. Treatment of anemia with ARANESP (darbepoetin alfa) improves health related quality of life (HRQOL) in patients with chronic kidney disease (CKD) [abstract]. Journal of the American Society of Nephrology 2002;13(Program & Abstracts):637A. [CENTRAL: CN‐00447983]
Tsubakihara 2003 {published data only}
-
- Tsubakihara Y, Iino Y, Akizawa T, Kosikawa S, KRN321 Study Group. Pharmacokinetics and safety of subcutaneous (SC) administration of KRN321 (darbepoetin alfa) in predialysis chronic kidney disease (CKD) patients [abstract no: SU‐PO781]. Journal of the American Society of Nephrology 2003;14(Nov):706A. [CENTRAL: CN‐00740543]
References to studies awaiting assessment
CORDATUS Study 2011 {published data only}
-
- Martinez‐Castelao A. C.E.R.A. once every 4 weeks (Q4W) corrects anaemia in chronic kidney disease (CKD) patients with low incidence of Hb values outside the target range [abstract no: OSu057]. NDT Plus 2010;3(Suppl 3):iii298. [EMBASE: 70484207]
-
- Roger S. C.E.R.A. once every 4 weeks (Q4W) corrects anaemia in patients with chronic kidney disease (CKD) not on dialysis and demonstrates comparable safety to darbepoetin alfa [abstract no: 202]. Nephrology 2010;15(Suppl 4):79‐80. [EMBASE: 70467206]
-
- Roger SD. C.E.R.A. once every 4 weeks (Q4W) corrects anaemia in patients with chronic kidney disease (CKD) not on dialysis and demonstrates comparable safety to darbepoetin alfa weekly (QW) or once every 2 weeks (Q2W) [abstract no: Sa535]. NDT Plus 2010;3(Suppl 3):iii219‐iii220. [EMBASE: 70484001]
Forni 2013 {published data only}
Gurevich 2010 {published data only}
-
- Gurevich A, Yampolskiy A, Timokhovskaya G, Tang H, Polu K, Duliege AM. Efficacy and tolerability of once‐monthly Hematide (peginesatide) in patients who are undergoing chronic hemodialysis and not on ESA treatment [abstract no: Sa510]. NDT Plus 2010;3(Suppl 3):iii209‐iii210. [EMBASE: 70483976]
Martinez 2006 {published data only}
-
- Martinez F, Mamzer M, Pillebout E, Tricot L, Legendre C, Thervet E. Comparison between 2 erythropeitic regimens during the initial period after renal transplantation [abstract no: PUB195]. Journal of the American Society of Nephrology 2006;17(Abstracts):857A. [CENTRAL: CN‐00774106]
Patel 2012 {published data only}
-
- Patel M, Thimons DG, Winston JL, Langholff W, McGowan T. An open‐label, randomized, multicenter, controlled study of epoetin alfa for the treatment of anemia of chronic kidney disease in the long term care setting. Journal of the American Medical Directors Association 2012;13(3):244‐8. [MEDLINE: ] - PubMed
PEARL 1 2013 {published data only}
-
- Macdougall IC, Provenzano R, Sharma A, Spinowitz BS, Schmidt RJ, Pergola PE, et al. Peginesatide for anemia in patients with chronic kidney disease not receiving dialysis. New England Journal of Medicine 2013;368(4):320‐32. [MEDLINE: ] - PubMed
PEARL 2 2013 {published data only}
-
- Macdougall IC, Provenzano R, Sharma A, Spinowitz BS, Schmidt RJ, Pergola PE, et al. Peginesatide for anemia in patients with chronic kidney disease not receiving dialysis. New England Journal of Medicine 2013;368(4):320‐32. [MEDLINE: ] - PubMed
References to ongoing studies
Besarab 2006 {published data only}
-
- Besarab A, Canaud B, Francisco AL, Kerr P, Locatelli F, Lok CE, et al. Randomized comparison of IV C.E.R.A. (Continuous Erythropoietin Receptor Activator) and Darbepoetin Alfa (DA) at extended administration intervals for the maintenance of Hb levels in patients with CKD on dialysis: rationale and design [abstract no: PUB377]. Journal of the American Society of Nephrology 2006;17(Abstracts):896A. [CENTRAL: CN‐00740564]
ISRCTN89787518 {published data only}
-
- Dedi R. Randomised controlled trial of maintaining low serum ferritin levels in patients receiving darbepoetin (Aranesp®). controlled‐trials.com/ISRCTN89787518 (accessed 3 January 2014).
Ito 2011 {published data only}
-
- Ito T. The effect of darbepoetin for renal anemia and iron kinetics in chronic kidney disease (CKD) patients. apps.who.int/trialsearch/Trial.aspx?TrialID=JPRN‐UMIN000005068 (accessed 3 January 2014).
NCT00121602 {published data only}
-
- NCT00121602. Randomized, double‐blind, equivalence study of the efficacy of darbepoetin alfa manufactured by serum free bioreactor technology and darbepoetin alfa manufactured by roller‐bottle technology for the treatment of anemia in patients with chronic kidney disease receiving hemodialysis. www.clinicaltrials.gov/ct2/show/NCT00121602 (accessed 3 January 2014).
NCT00436748 {published data only}
-
- NCT00436748. A multi‐center, double‐blind, randomized study evaluating de novo weekly and once every two week darbepoetin alfa dosing for the correction of anemia in pediatric subjects with chronic kidney disease receiving and not receiving dialysis. www.clinicaltrials.gov/ct2/show/NCT00436748 (accessed 3 January 2014).
NCT00442702 {published data only}
-
- NCT00442702. An open‐label, randomized, multi‐center, parallel group non‐inferiority study of subcutaneous injections of RO0503821 given once monthly vs. darbepoetin alfa given according to local label in patients with chronic kidney disease who are not on dialysis. www.clinicaltrials.gov/show/NCT00442702 (accessed 3 January 2014).
NCT00559273 {published data only}
-
- NCT00559273. An open‐label, randomized, multicenter, parallel‐group study to demonstrate correction of anemia using once every 4 weeks subcutaneous injections of Mircera in patients with chronic kidney disease who are not on dialysis. www.clinicaltrials.gov/ct2/show/NCT00559273 (accessed 3 January 2014).
NCT00605345 {published data only}
-
- NCT00605345. An open label randomised controlled study to compare the efficacy, safety and tolerability of once‐monthly administration of subcutaneous mircera versus darbepoetin alfa for the maintenance of haemoglobin levels in renal transplant recipients with chronic renal anaemia. www.clinicaltrials.gov/ct2/show/NCT00605345 (accessed 16 January 2014).
NCT00717821 {published data only}
-
- NCT00717821. A randomized, controlled, open label, french multicenter parallel group study to compare the hemoglobin maintenance with once monthly administration of mircera versus epoetin beta or darbepoetin alfa in patients with chronic kidney disease on hemodialysis. www.clinicaltrials.gov/ct2/show/NCT00717821 (accessed 3 January 2014).
NCT00773513 {published data only}
-
- NCT00773513. A randomized, open label study to assess all‐cause mortality and cardiovascular morbidity in patients with chronic kidney disease on dialysis and those not on renal replacement therapy under treatment with Mircera or reference ESAs. www.clinicaltrials.gov/ct2/show/NCT00773513 (accessed 3 January 2014).
NCT00925587 {published data only}
-
- NCT00925587. A multicenter, randomised, double‐blind study comparing de novo once monthly and once every 2 week darbepoetin alfa dosing for the correction of anemia in subjects with chronic kidney disease not receiving dialysis. www.clinicaltrials.gov/ct2/show/NCT00925587 (accessed 3 January 2014).
NCT01306409 {published data only}
-
- NCT01306409. Neocytolysis in the treatment of renal anemia with erythropoieses stimulating agents. www.clinicaltrials.gov/ct2/show/NCT01306409 (accessed 3 January 2014).
STIMULATE Study 2011 {published data only}
-
- NCT00364845. A randomised single‐blind study to improve health‐related quality of life as measured by the SF‐36 vitality score by correcting anemia with aranesp (darbepoetin alfa) in the elderly. www.clinicaltrials.gov/ct2/show/NCT00364845 (accessed 3 January 2014).
Additional references
Astor 2006
-
- Astor BC, Coresh J, Heiss G, Pettitt D, Sarnak MJ. Kidney function and anemia as risk factors for coronary heart disease and mortality: the Atherosclerosis Risk in Communities (ARIC) Study. American Heart Journal 2006;151(2):492‐500. [MEDLINE: ] - PubMed
Besarab 1998
-
- Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. New England Journal of Medicine 1998;339(9):584‐90. [MEDLINE: ] - PubMed
Drueke 2006
-
- Drueke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. New England Journal of Medicine 2006;355(20):2071‐84. [MEDLINE: ] - PubMed
EBPG 2004
-
- Locatelli F, Aljama P, Barany P, Canaud B, Carrera F, Eckardt KU, et al. Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrology Dialysis Transplantation 2004;19 Suppl 2:ii1‐47. [MEDLINE: ] - PubMed
Finkelstein 2009
Gandra 2010
-
- Gandra SR, Finkelstein FO, Bennett AV, Lewis EF, Brazg T, Martin ML. Impact of erythropoiesis‐stimulating agents on energy and physical function in nondialysis CKD patients with anemia: a systematic review. American Journal of Kidney Diseases 2010;55(3):519‐34. [MEDLINE: ] - PubMed
Gerson 2004
-
- Gerson A, Hwang W, Fiorenza J, Barth K, Kaskel F, Weiss L, et al. Anemia and health‐related quality of life in adolescents with chronic kidney disease. American Journal of Kidney Diseases 2004;44(6):1017‐23. [MEDLINE: ] - PubMed
Glassock 2009
-
- Glassock RJ, Pecoits‐Filho R, Barberato SH. Left ventricular mass in chronic kidney disease and ESRD. Clinical Journal of The American Society of Nephrology: CJASN 2009;4 Suppl 1:S79‐91. [MEDLINE: ] - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Johansen 2010
-
- Johansen KL, Finkelstein FO, Revicki DA, Gitlin M, Evans C, Mayne TJ. Systematic review and meta‐analysis of exercise tolerance and physical functioning in dialysis patients treated with erythropoiesis‐stimulating agents. American Journal of Kidney Diseases 2010;55(3):535‐48. [MEDLINE: ] - PubMed
KDIGO 2012
-
- Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney International ‐ Supplement 2012;2:279‐335.
KDOQI 2006
-
- KDOQI. National Kidney Foundation. Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease in Adults. American Journal of Kidney Diseases 2006;47(5 Suppl 3):S16‐85. [MEDLINE: ] - PubMed
Levin 1999
-
- Levin A, Thompson CR, Ethier J, Carlisle EJ, Tobe S, Mendelssohn D, et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. American Journal of Kidney Diseases 1999;34(1):125‐34. [MEDLINE: ] - PubMed
Locatelli 2000
-
- Locatelli F, Olivares J, Walker R, Wilkie M, the European/Australian NESP 980202 Study Group. Novel erythropoiesis stimulating protein (NESP) administered subcutaneously corrects anemia in subjects with chronic renal insufficiency (CRI) when administered at a reduced frequency compared with recombinant human erythropoietin (r‐HuEPO) [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):283A. [CENTRAL: CN‐00626023]
Locatelli 2004a
-
- Locatelli F, Pisoni RL, Combe C, Bommer J, Andreucci VE, Piera L, et al. Anaemia in haemodialysis patients of five European countries: association with morbidity and mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrology Dialysis Transplantation 2004;19(1):121‐32. [MEDLINE: ] - PubMed
Macdougall 2000
-
- Macdougall IC. Novel erythropoiesis stimulating protein. Seminars in Nephrology 2000;20(4):375‐81. [MEDLINE: ] - PubMed
NESP 2001
-
- Aljama P, Bommer J, Canaud B, Carrera F, Eckardt KU, Horl WH, et al. Practical guidelines for the use of NESP in treating renal anaemia. Nephrology Dialysis Transplantation 2001;16 Suppl 3:22‐8. [MEDLINE: ] - PubMed
Palmer 2010
-
- Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Tonelli M, Garg AX, et al. Meta‐analysis: erythropoiesis‐stimulating agents in patients with chronic kidney disease. Annals of Internal Medicine 2010;153(1):23‐33. [MEDLINE: ] - PubMed
Parfrey 2009
Pfeffer 2009
-
- Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, Zeeuw D, Eckardt KU, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. New England Journal of Medicine 2009;361(21):2019‐32. [MEDLINE: ] - PubMed
Phrommintikul 2007
-
- Phrommintikul A, Haas SJ, Elsik M, Krum H. Mortality and target haemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta‐analysis. Lancet 2007;369(9559):381‐8. [MEDLINE: ] - PubMed
Singh 2006
-
- Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, et al. Correction of anemia with epoetin alfa in chronic kidney disease. New England Journal of Medicine 2006;355(20):2085‐98. [MEDLINE: ] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

