Pharmacological interventions for prevention or treatment of postoperative pain in people undergoing laparoscopic cholecystectomy
- PMID: 24683057
- PMCID: PMC11086628
- DOI: 10.1002/14651858.CD008261.pub2
Pharmacological interventions for prevention or treatment of postoperative pain in people undergoing laparoscopic cholecystectomy
Abstract
Background: While laparoscopic cholecystectomy is generally considered less painful than open surgery, pain is one of the important reasons for delayed discharge after day-surgery and overnight stay following laparoscopic cholecystectomy. The safety and effectiveness of different pharmacological interventions such as non-steroidal anti-inflammatory drugs, opioids, and anticonvulsant analgesics in people undergoing laparoscopic cholecystectomy is unknown.
Objectives: To assess the benefits and harms of different analgesics in people undergoing laparoscopic cholecystectomy.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, Science Citation Index Expanded, and the World Health Organization International Clinical Trials Registry Platform portal (WHO ICTRP) to March 2013 to identify randomised clinical trials of relevance to this review.
Selection criteria: We considered only randomised clinical trials (irrespective of language, blinding, or publication status) comparing different pharmacological interventions with no intervention or inactive controls for outcomes related to benefit in this review. We considered comparative non-randomised studies with regards to treatment-related harms. We also considered trials that compared one class of drug with another class of drug for this review.
Data collection and analysis: Two review authors collected the data independently. We analysed the data with both fixed-effect and random-effects models using Review Manager 5 analysis. For each outcome, we calculated the risk ratio (RR) or mean difference (MD) with 95% confidence intervals (CI).
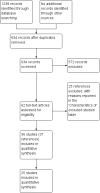
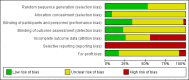
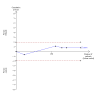
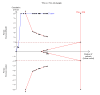
Main results: We included 25 trials with 2505 participants randomised to the different pharmacological agents and inactive controls. All the trials were at unclear risk of bias. Most trials included only low anaesthetic risk people undergoing elective laparoscopic cholecystectomy. Participants were allowed to take additional analgesics as required in 24 of the trials. The pharmacological interventions in all the included trials were aimed at preventing pain after laparoscopic cholecystectomy. There were considerable differences in the pharmacological agents used and the methods of administration. The estimated effects of the intervention on the proportion of participants who were discharged as day-surgery, the length of hospital stay, or the time taken to return to work were imprecise in all the comparisons in which these outcomes were reported (very low quality evidence). There was no mortality in any of the groups in the two trials that reported mortality (183 participants, very low quality evidence). Differences in serious morbidity outcomes between the groups were imprecise across all the comparisons (very low quality evidence). None of the trials reported patient quality of life or time taken to return to normal activity. The pain at 4 to 8 hours was generally reduced by about 1 to 2 cm on the visual analogue scale of 1 to 10 cm in the comparisons involving the different pharmacological agents and inactive controls (low or very low quality evidence). The pain at 9 to 24 hours was generally reduced by about 0.5 cm (a modest reduction) on the visual analogue scale of 1 to 10 cm in the comparisons involving the different pharmacological agents and inactive controls (low or very low quality evidence).
Authors' conclusions: There is evidence of very low quality that different pharmacological agents including non-steroidal anti-inflammatory drugs, opioid analgesics, and anticonvulsant analgesics reduce pain scores in people at low anaesthetic risk undergoing elective laparoscopic cholecystectomy. However, the decision to use these drugs has to weigh the clinically small reduction in pain against uncertain evidence of serious adverse events associated with many of these agents. Further randomised clinical trials of low risk of systematic and random errors are necessary. Such trials should include important clinical outcomes such as quality of life and time to return to work in their assessment.
Conflict of interest statement
None known.
Figures
































Update of
- doi: 10.1002/14651858.CD008261
References
References to studies included in this review
Abdulla 2012 {published data only}
-
- Abdulla S, Eckhardt R, Netter U, Abdulla W. A randomized, double‐blind, controlled trial on non‐opioid analgesics and opioid consumption for postoperative pain relief after laparoscopic cholecystectomy. Acta Anaesthesiologica Belgica 2012;63(1):43‐50. - PubMed
Agarwal 2008 {published data only}
-
- Agarwal A, Gautam S, Gupta D, Agarwal S, Singh PK, Singh U. Evaluation of a single preoperative dose of pregabalin for attenuation of postoperative pain after laparoscopic cholecystectomy. British Journal of Anaesthesia 2008;101(5):700‐4. - PubMed
Akaraviputh 2009 {published data only}
Akarsu 2012 {published data only}
-
- Akarsu T, Tur H, Bolat C, Ozkaynak I. Comparison of pre‐emptive pregabalin with placebo and diclofenac combination for postoperative analgesia and cognitive functions after laparoscopic cholecystectomy. Turkiye Klinikleri Journal of Medical Sciences 2012;32(4):963‐70.
Akinci 2008 {published data only}
-
- Akinci SB, Ayhan B, Aycan IO, Tirnaksiz B, Basgul E, Abbasoglu O, et al. The postoperative analgesic efficacy of intraperitoneal tramadol compared to normal saline or intravenous tramadol in laparoscopic cholecystectomy. European Journal of Anaesthesiology 2008;25(5):375‐81. - PubMed
Balaban 2012 {published data only}
-
- Balaban F, Yagar S, Ozgok A, Koc M, Gullapoglu H. A randomized, placebo‐controlled study of pregabalin for postoperative pain intensity after laparoscopic cholecystectomy. Journal of Clinical Anesthesia 2012;24(3):175‐8. - PubMed
Belzarena 1998 {published data only}
-
- Belzarena SD. Intravenous tenoxicam for postoperative pain relief after laparoscopic cholecystectomy. A comparison among placebo, 20 and 40 mg of tenoxicam [Tenoxicam venoso para analgesia pos operatoria em colecistectomia videolaparascopica. Comparacao entre placebo, 20 e 40 mg de tenoxicam]. Revista Brasileira De Anestesiologia 1998;48(1):7‐13.
Cheng 2004 {published data only}
-
- Cheng PGB, Lim MJ, Onsiong MK, Chiu KYW, Chan MK, Li KWM, et al. Celecoxib premedication in post‐operative analgesia for laparoscopic cholecystectomy. Acute Pain 2004;6(1):23‐8.
Chung 2004 {published data only}
-
- Chung F, Tong D, Miceli PC, Reiz J, Harsanyi Z, Darke AC, et al. Controlled‐release codeine is equivalent to acetaminophen plus codeine for post‐cholecystectomy analgesia. Canadian Journal of Anaesthesia 2004;51(3):216‐21. - PubMed
Dong 2003 {published data only}
-
- Dong FT, Yang YL, Guo J. Postoperative analgesia with lornoxicam in patients undergoing laparoscopic cholecystectomy. Academic Journal of Kunming Medical College 2003;24(2):71‐3.
Fanelli 2008 {published data only}
-
- Fanelli G, Ghisi D, Berti M, Troglio R, Ortu A, Consigli C, et al. Preoperative administration of controlled‐release oxycodone as a transition opioid for total intravenous anaesthesia in pain control after laparoscopic cholecystectomy. Surgical Endoscopy 2008;22(10):2220‐8. - PubMed
Forse 1996 {published data only}
Gilron 2009 {published data only}
-
- Gilron I, Orr E, Tu D, Mercer CD, Bond D. A randomized, double‐blind, controlled trial of perioperative administration of gabapentin, meloxicam and their combination for spontaneous and movement‐evoked pain after ambulatory laparoscopic cholecystectomy. Anesthesia and Analgesia 2009;108(2):623‐30. - PubMed
Gomez‐Vazquez 2012 {published data only}
-
- Gomez‐Vazquez ME, Hernandez‐Salazar E, Novelo‐Otanez JD, Cabrera‐Pivaral CE, Davalos‐Rodriguez IP, Salazar‐Paramo M. Effect of endovenous morphine vs. ketorolac on proinflammatory cytokines during postoperative analgesia in laparoscopic cholecystectomy. Cirugia y Cirujanos 2012;80(1):56‐62. - PubMed
Horattas 2004 {published data only}
-
- Horattas MC, Evans S, Sloan‐Stakleff KD, Lee C, Snoke JW. Does preoperative rofecoxib (Vioxx) decrease postoperative pain with laparoscopic cholecystectomy?. American Journal of Surgery 2004;188(3):271‐6. - PubMed
Ji 2010 {published data only}
-
- Ji FH, Jin X, Yang JP, Zan LL. Analgesic effect of parecoxib and flurbiprofen axetil for patients undergoing laparoscopic cholecystectomy and their influences on platelet aggregation. Chinese Medical Journal 2010;123(12):1607‐9. - PubMed
Joshi 2004 {published data only}
-
- Gan TJ, Joshi GP, Viscusi E, Cheung RY, Dodge W, Fort JG, et al. Preoperative parenteral parecoxib and follow‐up oral valdecoxib reduce length of stay and improve quality of patient recovery after laparoscopic cholecystectomy surgery. Anesthesia and Analgesia 2004;98(6):1665‐73. - PubMed
-
- Joshi GP, Viscusi ER, Gan TJ, Minkowitz H, Cippolle M, Schuller R, et al. Effective treatment of laparoscopic cholecystectomy pain with intravenous followed by oral COX‐2 specific inhibitor. Anesthesia and Analgesia 2004;98(2):336‐42. - PubMed
Karakoc 2011 {published data only}
-
- Karakoc F, Akcaboy EY, Akcaboy ZN, Gogus N. The effects of intravenous dexketoprofen trometamol on postoperative analgesia and morphine consumption undergoing laparoscopic cholecystectomy. Regional Anesthesia and Pain Medicine 2011;2:E165‐E166.
Lane 1996 {published data only}
-
- Lane GE, Lathrop JC, Boysen DA, Lane RC. Effect of intramuscular intraoperative pain medication on narcotic usage after laparoscopic cholecystectomy. American Surgeon 1996;62(11):907‐10. - PubMed
Liu 1993 {published data only}
-
- Liu J, Ding Y, White PF, Feinstein R, Shear JM. Effects of ketorolac on postoperative analgesia and ventilatory function after laparoscopic cholecystectomy. Anesthesia and Analgesia 1993;76(5):1061‐6. - PubMed
Mebazaa 2008 {published data only}
-
- Mebazaa MS, Frikha N, Hammouda NB, Mestiri T, Mestiri H, Khalfallah T, et al. Postoperative analgesia after laparoscopic cholecystectomy: comparison of the preoperative administration of celecoxib with paracetamol?. Tunisie Medicale 2008;86(10):869‐73. - PubMed
Muñoz 2002 {published data only}
-
- Muñoz HR, Guerrero ME, Brandes V, Cortínez LI. Effect of timing of morphine administration during remifentanil‐based anaesthesia on early recovery from anaesthesia and postoperative pain. British Journal of Anaesthesia 2002;88(6):814‐8. - PubMed
Munro 1998 {published data only}
-
- Munro FJ, Young SJ, Broome IJ, Robb HM, Wardall GJ. Intravenous tenoxicam for analgesia following laparoscopic cholecystectomy. Anaesthesia and Intensive Care 1998;26(1):56‐60. - PubMed
Nesek‐Adam 2012 {published data only}
-
- Nesek‐Adam V, Grizelj‐Stojcic E, Mrsic V, Rasic Z, Schwarz D. Preemptive use of diclofenac in combination with ketamine in patients undergoing laparoscopic cholecystectomy: a randomized, double‐blind, placebo‐controlled study. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2012;22(3):232‐8. - PubMed
Pandey 2004 {published data only}
-
- Pandey CK, Priye S, Singh S, Singh U, Singh RB, Singh PK. Preemptive use of gabapentin significantly decreases postoperative pain and rescue analgesic requirements in laparoscopic cholecystectomy. Canadian Journal of Anaesthesia 2004;51(4):358‐63. - PubMed
Peng 2010 {published data only}
-
- Peng PW, Li C, Farcas E, Haley A, Wong W, Bender J, et al. Use of low‐dose pregabalin in patients undergoing laparoscopic cholecystectomy. British Journal of Anaesthesia 2010;105(2):155‐61. - PubMed
Puura 2006 {published data only}
-
- Puura A, Puolakka P, Rorarius M, Salmelin R, Lindgren L. Etoricoxib pre‐medication for post‐operative pain after laparoscopic cholecystectomy. Acta Anaesthesiologica Scandinavica 2006;50(6):688‐93. - PubMed
Salihoglu 2009 {published data only}
-
- Salihoglu Z, Yildirim M, Demiroluk S, Kaya G, Karatas A, Ertem M, et al. Evaluation of intravenous paracetamol administration on postoperative pain and recovery characteristics in patients undergoing laparoscopic cholecystectomy. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2009;19(4):321‐3. - PubMed
Sandhu 2011 {published data only}
-
- Sandhu T, Paiboonworachat S, Ko‐iam W. Effects of preemptive analgesia in laparoscopic cholecystectomy: a double‐blind randomized controlled trial. Surgical Endoscopy 2011;25(1):23‐7. - PubMed
Sarakatsianou 2013 {published data only}
-
- Sarakatsianou C, Theodorou E, Georgopoulou S, Stamatiou G, Tzovaras G. Effect of pre‐emptive pregabalin on pain intensity and postoperative morphine consumption after laparoscopic cholecystectomy. Surgical Endoscopy 2013;27(7):2504‐11. - PubMed
Schuster 2005 {published data only}
-
- Schuster R, Stewart D, Schuster L, Greaney G, Waxman K. Preoperative oral rofecoxib and postoperative pain in patients after laparoscopic cholecystectomy: a prospective, randomized, double‐blinded, placebo‐controlled trial. American Surgeon 2005;71(10):827‐9. - PubMed
Sen 2010 {published data only}
-
- Sen M, Inan A, Sert H, Akpinar A, Dener C. Preemptive use of etofenamate in laparoscopic cholecystectomy: a randomized, placebo‐controlled, double‐blind study. European Journal of General Medicine 2010;7(1):50‐5.
Wilson 1994 {published data only}
-
- Wilson YG, Rhodes M, Ahmed R, Daugherty M, Cawthorn SJ, Armstrong CP. Intramuscular diclofenac sodium for postoperative analgesia after laparoscopic cholecystectomy: a randomised, controlled trial. Surgical Laparoscopy & Endoscopy 1994;4(5):340‐4. - PubMed
Yeh 2004 {published data only}
-
- Yeh CC, Wu CT, Lee MS, Yu JC, Yang CP, Lu CH, et al. Analgesic effects of preincisional administration of dextromethorphan and tenoxicam following laparoscopic cholecystectomy. Acta Anaesthesiologica Scandinavica 2004;48(8):1049‐53. - PubMed
Zajaczkowska 2004 {published data only}
-
- Zajaczkowska R, Wnek W, Wordliczek J, Dobrogowski J. Peripheral opioid analgesia in laparoscopic cholecystectomy. Regional Anesthesia and Pain Medicine 2004;29(5):424‐9. - PubMed
References to studies excluded from this review
Aftab 2008 {published data only}
-
- Aftab S, Rashdi S. Comparison of intravenous ketorolac with diclofenac for postoperative analgesia. Journal of Surgery Pakistan 2008;13(2):62‐6.
Akca 2004 {published data only}
-
- Akca T, Colak T, Kanik A, Yaylak F, Caglikulekci M, Aydin S. The effect of preoperative intravenous use of tenoxicam: a prospective, double‐blind, placebo‐controlled study. Journal of Investigative Surgery 2004;17(6):333‐8. - PubMed
Bajaj 2004 {published data only}
-
- Bajaj P, Ballary CC, Dongre NA, Baliga VP, Desai AA. Role of parecoxib in pre‐emptive analgesia: comparison of the efficacy and safety of pre‐ and postoperative parecoxib in patients undergoing general surgery. Journal of the Indian Medical Association 2004;102(5):272‐8. - PubMed
Boccara 2005 {published data only}
-
- Boccara G, Chaumeron A, Pouzeratte Y, Mann C. The preoperative administration of ketoprofen improves analgesia after laparoscopic cholecystectomy in comparison with propacetamol or postoperative ketoprofen. British Journal of Anaesthesia 2005;94(3):347‐51. - PubMed
Collard 2007 {published data only}
-
- Collard V, Mistraletti G, Taqi A, Asenjo JF, Feldman LS, Fried GM, et al. Intraoperative esmolol infusion in the absence of opioids spares postoperative fentanyl in patients undergoing ambulatory laparoscopic cholecystectomy. Anesthesia and Analgesia 2007;105(5):1255‐62. - PubMed
Elhakim 2000 {published data only}
-
- Elhakim M, Amine H, Kamel S, Saad F. Effects of intraperitoneal lidocaine combined with intravenous or intraperitoneal tenoxicam on pain relief and bowel recovery after laparoscopic cholecystectomy. Acta Anaesthesiologica Scandinavica 2000;44(8):929‐33. - PubMed
Gan 2004 {published data only}
-
- Gan TJ, Joshi GP, Zhao SZ, Hanna DB, Cheung RY, Chen C. Presurgical intravenous parecoxib sodium and follow‐up oral valdecoxib for pain management after laparoscopic cholecystectomy surgery reduces opioid requirements and opioid‐related adverse effects. Acta Anaesthesiologica Scandinavica 2004;48(9):1194‐207. - PubMed
Hernandez‐Palazon 2003 {published data only}
-
- Hernandez‐Palazon J, Tortosa JA, Rosa VN, Gimenez‐Viudes J, Ramirez G, Robles R. Intraperitoneal application of bupivacaine plus morphine for pain relief after laparoscopic cholecystectomy. European Journal of Anaesthesiology 2003;20(11):891‐6. - PubMed
Kocaayan 2007 {published data only}
-
- Kocaayan E, Ozkardeşler S, Ozzeybek D, Bayindir S, Akan M. Comparison of effects of preoperatively administered lornoxicam and tenoxicam on morphine consumption after laparoscopic cholecystectomy. European Journal of Anaesthesiology 2007;24(8):714‐9. - PubMed
Koch 2008 {published data only}
-
- Koch S, Ahlburg P, Spangsberg N, Brock B, Tonnesen E, Nikolajsen L. Oxycodone vs. fentanyl in the treatment of early post‐operative pain after laparoscopic cholecystectomy: a randomised double‐blind study. Acta Anaesthesiologica Scandinavica 2008;52(6):845‐50. - PubMed
Kokki 2012 {published data only}
-
- Kokki M, Broms S, Eskelinen M, Neuvonen PJ, Halonen T, Kokki H. The analgesic concentration of oxycodone with co‐administration of paracetamol ‐ a dose‐finding study in adult patients undergoing laparoscopic cholecystectomy. Basic & Clinical Pharmacology & Toxicology 2012;111(6):391‐5. - PubMed
Matkap 2011 {published data only}
-
- Matkap E, Bedirli N, Akkaya T, Gümü H. Preincisional local infiltration of tramadol at the trocar site versus intravenous tramadol for pain control after laparoscopic cholecystectomy. Journal of Clinical Anesthesia 2011;23(3):197‐201. - PubMed
Naguib 1998 {published data only}
-
- Naguib M, Seraj M, Attia M, Samarkandi AH, Seet M, Jaroudi R. Perioperative antinociceptive effects of tramadol. A prospective, randomized, double‐blind comparison with morphine. Canadian Journal of Anaesthesia 1998;45(12):1168‐75. - PubMed
O'Hanlon 2002 {published data only}
-
- O'Hanlon DM, Colbert S, Ragheb J, McEntee GP, Chambers F, Moriarty DC. Intraperitoneal pethidine versus intramuscular pethidine for the relief of pain after laparoscopic cholecystectomy: randomized trial. World Journal of Surgery 2002;26(12):1432‐6. - PubMed
Ozkocak 2002 {published data only}
-
- Ozkocak I, Kirdemir P, Rasa K, Aksu C, Gogus N. The comparison of preemptive intraperitoneal analgesic effects of tramadol, pethidine and bupivacaine. Anestezi Dergisi 2002;10(1):49‐52.
Sanchez‐Rodriguez 2010 {published data only}
-
- Sanchez‐Rodriguez PE, Fuentes‐Orozco C, Gonzalez‐Ojeda A. Effect of dexamethasone on postoperative symptoms in patients undergoing elective laparoscopic cholecystectomy: randomized clinical trial. World Journal of Surgery 2010;34(5):895‐900. - PubMed
Sozbilen 2007 {published data only}
-
- Sozbilen M, Yeniay L, Unalp O, Makay O, Pirim A, Ulukaya S, et al. Effects of ropivacaine on pain after laparoscopic cholecystectomy: a prospective, randomized study. Advances in Therapy 2007;24(2):247‐57. - PubMed
Stempin 2007 {published data only}
-
- Stempin S, Gajdosz R. Intraperitoneal morphine for prevention of postoperative shoulder pain after laparoscopic cholecystectomy. Anestezjologia Intensywna Terapia 2007;39(1):18‐20.
Tiippana 2008 {published data only}
-
- Tiippana E, Bachmann M, Kalso E, Pere P. Effect of paracetamol and coxib with or without dexamethasone after laparoscopic cholecystectomy. Acta Anaesthesiologica Scandinavica 2008;52(5):673‐80. - PubMed
Wininger 2010 {published data only}
-
- Wininger SJ, Miller H, Minkowitz HS, Royal MA, Ang RY, Breitmeyer JB, et al. A randomized, double‐blind, placebo‐controlled, multicenter, repeat‐dose study of two intravenous acetaminophen dosing regimens for the treatment of pain after abdominal laparoscopic surgery. Clinical Therapeutics 2010;32(14):2348‐69. - PubMed
Wu 1999 {published data only}
-
- Wu CT, Yu JC, Yeh CC, Liu ST, Li CY, Ho ST, et al. Preincisional dextromethorphan treatment decreases postoperative pain and opioid requirement after laparoscopic cholecystectomy. Anesthesia and Analgesia 1999;88(6):1331‐4. - PubMed
Wu 2005 {published data only}
-
- Wu CT, Borel CO, Lee MS, Yu JC, Liou HS, Yi HD, et al. The interaction effect of perioperative cotreatment with dextromethorphan and intravenous lidocaine on pain relief and recovery of bowel function after laparoscopic cholecystectomy. Anesthesia and Analgesia 2005;100(2):448‐53. - PubMed
Yamazaki 2003 {published data only}
-
- Yamazaki E, Murao K, Asai T, Matsumoto S, Shingu K. Comparison of analgesic effects of intravenous flurbprofen and suppository indomethacin after laparoscopic cholecystectomy. Masui. The Japanese Journal of Anesthesiology 2003;52(11):1186‐90. - PubMed
Zambouri 2002 {published data only}
-
- Zambouri A, Petropoulou P, Petra K, Ralli M, Douvantzi A, Papachristou D. Do early postoperative pain, nausea and vomiting really differ when remifentanil or fentanyl are used in laparoscopic cholecystectomy?. 10th World Society of Pain Clinicians of the International Pain Clinic; 2002 May 04‐08, Sardinia, Italy. World Society of Pain Clinicians, 2002:257‐63.
Zghidi 2011 {published data only}
-
- Zghidi SM, Jaoua H, Ghariani S, Saada S, Laabidi S, Khemiri K, et al. Effectiveness of dexamethasone in postoperative analgesia after laparoscopic cholecystectomy. Regional Anesthesia and Pain Medicine 2011;2:E278‐9.
References to studies awaiting assessment
Gan 2003 {published data only}
-
- Gan TJ, Joshi G, Viscusi E, Chen C, Cheung R. Postdischarge recovery experience after single presurgery does of IV parecoxib sodium, a novel COX‐2 inhibitor, followed by oral valdecoxib for pain after laparoscopic cholecystectomy. International Journal of Obstetrics & Gynecology 2003;83(3):23.
Additional references
Alexander 1987
-
- Alexander JI, Hull MG. Abdominal pain after laparoscopy: the value of a gas drain. British Journal of Obstetrics and Gynaecology 1987;94(3):267‐9. - PubMed
Argoff 2013
-
- Argoff CE. Recent management advances in acute postoperative pain. Pain Practice 2013 Aug 15 [Epub ahead of print]. - PubMed
Attili 1995
-
- Attili AF, Santis A, Capri R, Repice AM, Maselli S. The natural history of gallstones: the GREPCO experience. The GREPCO group. Hepatology 1995;21(3):655‐60. - PubMed
Ballal 2009
-
- Ballal M, David G, Willmott S, Corless DJ, Deakin M, Slavin JP. Conversion after laparoscopic cholecystectomy in England. Surgical Endoscopy 2009;23(10):2338‐44. - PubMed
Bates 1992
Bisgaard 2006
-
- Bisgaard T. Analgesic treatment after laparoscopic cholecystectomy: a critical assessment of the evidence. Anesthesiology 2006;104(4):835‐46. - PubMed
Brok 2008
-
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. - PubMed
Brok 2009
-
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. - PubMed
CTU 2011
-
- Copenhagen Trial Unit. TSA ‐ trial sequential analysis, 2011. ctu.dk/tsa/ (accessed 25 March 2014).
DeMets 1987
-
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Dolan 2009
-
- Dolan JP, Diggs BS, Sheppard BC, Hunter JG. The national mortality burden and significant factors associated with open and laparoscopic cholecystectomy: 1997‐2006. Journal of Gastrointestinal Surgery 2009;13(12):2292‐301. - PubMed
Egger 1997
Giger 2011
-
- Giger U, Ouaissi M, Schmitz SF, Krahenbuhl S, Krahenbuhl L. Bile duct injury and use of cholangiography during laparoscopic cholecystectomy. British Journal of Surgery 2011;98(3):391‐6. - PubMed
Gluud 2014
-
- Nikolova D, Klingenberg SL, Gluud C, Als‐Nielsen B, Bjelakovic G, Casazza G, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2014, Issue 2. Art. No.: LIVER.
Gottschling 2005
-
- Gottschling S, Meyer S, Krenn T, Reinhard H, Lothschuetz D, Nunold H, et al. Propofol versus midazolam/ketamine for procedural sedation in pediatric oncology. Journal of Pediatric Hematology/Oncology 2005;27(9):471‐6. - PubMed
GREPCO 1984
-
- GREPCO. Prevalence of gallstone disease in an Italian adult female population. Rome group for the epidemiology and prevention of cholelithiasis (GREPCO). American Journal of Epidemiology 1984;119(5):796‐805. - PubMed
GREPCO 1988
-
- GREPCO. The epidemiology of gallstone disease in Rome, Italy. Part i. Prevalence data in men. The Rome group for epidemiology and prevention of cholelithiasis (GREPCO). Hepatology 1988;8(4):904‐6. - PubMed
Gurusamy 2008a
Gurusamy 2008b
-
- Gurusamy K, Junnarkar S, Farouk M, Davidson BR. Meta‐analysis of randomized controlled trials on the safety and effectiveness of day‐case laparoscopic cholecystectomy. British Journal of Surgery 2008;95(2):161‐8. - PubMed
Gurusamy 2009
-
- Gurusamy KS, Gluud C, Nikolova D, Davidson BR. Assessment of risk of bias in randomized clinical trials in surgery. British Journal of Surgery 2009;96(4):342‐9. - PubMed
Gurusamy 2013
Gurusamy 2014
-
- Gurusamy KS, Nagendran M, Guerrini GP, Toon CD, Zinnuroglu M, Davidson BR. Intraperitoneal local anaesthetic instillation versus no intraperitoneal local anaesthetic instillation for laparoscopic cholecystectomy. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD007337.pub3] - DOI - PubMed
Halldestam 2004
-
- Halldestam I, Enell EL, Kullman E, Borch K. Development of symptoms and complications in individuals with asymptomatic gallstones. British Journal of Surgery 2004;91(6):734‐8. - PubMed
HES 2011
-
- HESonline. Hospital episode statistics. Main procedures and interventions: 3 character, 2011. www.hesonline.nhs.uk/Ease/servlet/ContentServer?siteID=1937&category... (accessed on 25 March 2014).
Higgins 2002
-
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ICH‐GCP 1997
-
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997.
Inturrisi 2002
-
- Inturrisi CE. Clinical pharmacology of opioids for pain. Clinical Journal of Pain 2002;18(4 Suppl):S3‐13. - PubMed
Kehlet 2005
-
- Kehlet H, Gray AW, Bonnet F, Camu F, Fischer HB, McCloy RF, et al. A procedure‐specific systematic review and consensus recommendations for postoperative analgesia following laparoscopic cholecystectomy. Surgical Endoscopy 2005;19(10):1396‐415. - PubMed
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Loizides 2014
Lundh 2012
Macaskill 2001
-
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. - PubMed
Martindale 2011
-
- Sweetman S (editor). Martindale: the complete drug reference (online version), 37th edition, 2011. www.pharmpress.com/product/MC_MART/martindale‐the‐complete‐drug‐reference (accessed 25 March 2014).
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. - PubMed
Newell 1992
-
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. - PubMed
Ng 2004
-
- Ng A, Swami A, Smith G, Robertson G, Lloyd DM. Is intraperitoneal levobupivacaine with epinephrine useful for analgesia following laparoscopic cholecystectomy? A randomized controlled trial. European Journal of Anaesthesiology 2004; Vol. 21, issue 8:653‐7. - PubMed
NIH 1992
-
- NIH. NIH consensus statement on gallstones and laparoscopic cholecystectomy, 1992. consensus.nih.gov/1992/1992GallstonesLaparoscopy090html.htm (accessed 25 March 2014). - PubMed
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Royle 2003
-
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Savovic 2012a
-
- Savovic J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1‐82. - PubMed
Savovic 2012b
-
- Savovic J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. - PubMed
Strasberg 1993
-
- Strasberg SM, Clavien PA. Overview of therapeutic modalities for the treatment of gallstone diseases. American Journal of Surgery 1993;165(4):420‐6. - PubMed
Thorlund 2009
-
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. - PubMed
Thorlund 2010
Thorlund 2011
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for trial sequential analysis (TSA), 2011. ctu.dk/tsa/files/tsa_manual.pdf (accessed 25 March 2014).
Todd 1996
-
- Todd KH, Funk JP. The minimum clinically important difference in physician‐assigned visual analog pain scores. Academic Emergency Medicine 1996;3(2):142‐6. [PUBMED: 8808375] - PubMed
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. - PubMed
Wetterslev 2009
Wood 2008
Yu 2003
-
- Yu HP, Hseu SS, Yien HW, Teng YH, Chan KH. Oral clonidine premedication preserves heart rate variability for patients undergoing laparoscopic cholecystectomy. Acta Anaesthesiologica Scandinavica 2003;47(2):185‐90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

