Transcriptome analysis of an mvp mutant reveals important changes in global gene expression and a role for methyl jasmonate in vernalization and flowering in wheat
- PMID: 24683181
- PMCID: PMC4036498
- DOI: 10.1093/jxb/eru102
Transcriptome analysis of an mvp mutant reveals important changes in global gene expression and a role for methyl jasmonate in vernalization and flowering in wheat
Abstract
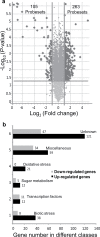
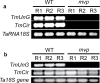
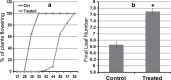
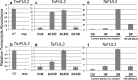
The einkorn wheat mutant mvp-1 (maintained vegetative phase 1) has a non-flowering phenotype caused by deletions including, but not limited to, the genes CYS, PHYC, and VRN1. However, the impact of these deletions on global gene expression is still unknown. Transcriptome analysis showed that these deletions caused the upregulation of several pathogenesis-related (PR) and jasmonate-responsive genes. These results suggest that jasmonates may be involved in flowering and vernalization in wheat. To test this hypothesis, jasmonic acid (JA) and methyl jasmonate (MeJA) content in mvp and wild-type plants was measured. The content of JA was comparable in all plants, whereas the content of MeJA was higher by more than 6-fold in mvp plants. The accumulation of MeJA was also observed in vernalization-sensitive hexaploid winter wheat during cold exposure. This accumulation declined rapidly once plants were deacclimated under floral-inductive growth conditions. This suggests that MeJA may have a role in floral transition. To confirm this result, we treated vernalization-insensitive spring wheat with MeJA. The treatment delayed flowering with significant downregulation of both TaVRN1 and TaFT1 genes. These data suggest a role for MeJA in modulating vernalization and flowering time in wheat.
Keywords: Biotic stress; flowering; maintained vegetative phase; methyl jasmonate; vernalization; wheat..
© The Author 2014. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures



Similar articles
-
The vernalization-induced long non-coding RNA VAS functions with the transcription factor TaRF2b to promote TaVRN1 expression for flowering in hexaploid wheat.Mol Plant. 2021 Sep 6;14(9):1525-1538. doi: 10.1016/j.molp.2021.05.026. Epub 2021 May 27. Mol Plant. 2021. PMID: 34052392
-
Expression of vernalization responsive genes in wheat is associated with histone H3 trimethylation.Mol Genet Genomics. 2012 Jul;287(7):575-90. doi: 10.1007/s00438-012-0701-0. Epub 2012 Jun 10. Mol Genet Genomics. 2012. PMID: 22684814
-
A genetic network of flowering-time genes in wheat leaves, in which an APETALA1/FRUITFULL-like gene, VRN1, is upstream of FLOWERING LOCUS T.Plant J. 2009 May;58(4):668-81. doi: 10.1111/j.1365-313X.2009.03806.x. Epub 2009 Jan 28. Plant J. 2009. PMID: 19175767 Free PMC article.
-
The molecular basis of vernalization-induced flowering in cereals.Trends Plant Sci. 2007 Aug;12(8):352-7. doi: 10.1016/j.tplants.2007.06.010. Epub 2007 Jul 12. Trends Plant Sci. 2007. PMID: 17629542 Review.
-
Methyl Jasmonate: An Alternative for Improving the Quality and Health Properties of Fresh Fruits.Molecules. 2016 May 31;21(6):567. doi: 10.3390/molecules21060567. Molecules. 2016. PMID: 27258240 Free PMC article. Review.
Cited by
-
A role for jasmonates in the release of dormancy by cold stratification in wheat.J Exp Bot. 2016 May;67(11):3497-508. doi: 10.1093/jxb/erw172. Epub 2016 May 2. J Exp Bot. 2016. PMID: 27140440 Free PMC article.
-
Transcriptomic analysis of methyl jasmonate treatment reveals gene networks involved in drought tolerance in pearl millet.Sci Rep. 2022 Mar 25;12(1):5158. doi: 10.1038/s41598-022-09152-6. Sci Rep. 2022. PMID: 35338214 Free PMC article.
-
Manipulation of Barley Development and Flowering Time by Exogenous Application of Plant Growth Regulators.Front Plant Sci. 2022 Jan 3;12:694424. doi: 10.3389/fpls.2021.694424. eCollection 2021. Front Plant Sci. 2022. PMID: 35046965 Free PMC article.
-
Association mapping of autumn-seeded rye (Secale cereale L.) reveals genetic linkages between genes controlling winter hardiness and plant development.Sci Rep. 2022 Apr 6;12(1):5793. doi: 10.1038/s41598-022-09582-2. Sci Rep. 2022. PMID: 35388069 Free PMC article.
-
Repression of Lateral Organ Boundary Genes by PENNYWISE and POUND-FOOLISH Is Essential for Meristem Maintenance and Flowering in Arabidopsis.Plant Physiol. 2015 Nov;169(3):2166-86. doi: 10.1104/pp.15.00915. Epub 2015 Sep 28. Plant Physiol. 2015. PMID: 26417006 Free PMC article.
References
-
- Albrechtova J, Ullmann J. 1994. Methyl jasmonate inhibits growth and flowering in Chenopodium rubrum . Biologia Plantarum 36, 317–319
-
- Avanci N, Luche D, Goldman G, Goldman M. 2010. Jasmonates are phytohormones with multiple functions, including plant defense and reproduction. Genetics and Molecular Research 9, 484–505 - PubMed
-
- Beale M, Ward J. 1998. Jasmonates: key players in the plant defense. Natural Product Reports 15, 533–548 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

