Molecular mechanisms of TLR2-mediated antigen cross-presentation in dendritic cells
- PMID: 24683188
- PMCID: PMC3993050
- DOI: 10.4049/jimmunol.1302850
Molecular mechanisms of TLR2-mediated antigen cross-presentation in dendritic cells
Abstract
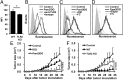
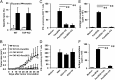
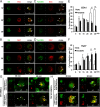
Cross-presentation is a key function of dendritic cells (DCs), which present exogenous Ags on MHC class I molecules to prime CTL responses. The effects of TLR triggering on the cross-presentation of exogenous Ags by DCs remain unclear. In this study, we used synthetic dipalmitoylated peptides and TLR2 agonist-conjugated peptides as models to elucidate the mechanisms of TLR2-mediated cross-presentation. We observed that the internalization of dipalmitoylated peptides by bone marrow-derived DCs was facilitated by TLR2 via clathrin-mediated endocytosis. The administration of these dipalmitoylated peptide-pulsed bone marrow-derived DCs eliminated established tumors through TLR2 signaling. We further demonstrated that the induction of Ag-specific CTL responses and tumor regression by dipalmitoylated peptides was TAP independent. In addition, presentation of dipalmitoylated peptides by MHC class I molecules was blocked in the presence of an endosomal acidification inhibitor (chloroquine) or a lysosomal degradation inhibitor (Z-FL-COCHO). The endocytosed dipalmitoylated peptide also passed rapidly from early endosome Ag-1-positive endosomes to RAS-related GTP-binding protein 7 (Rab7)-associated late endosomes compared with their nonlipidated counterparts. Furthermore, we found that dipalmitoylated peptide-upregulated Rab7 expression correlated with Ag presentation via the TLR2/MyD88 pathway. Both JNK and ERK signaling pathways are required for upregulation of Rab7. In summary, our data suggest that TLR2-mediated cross-presentation occurs through the upregulation of Rab7 and a TAP-independent pathway that prime CTL responses.
Figures


Similar articles
-
A marked reduction in priming of cytotoxic CD8+ T cells mediated by stress-induced glucocorticoids involves multiple deficiencies in cross-presentation by dendritic cells.J Immunol. 2011 Jan 1;186(1):183-94. doi: 10.4049/jimmunol.1001737. Epub 2010 Nov 22. J Immunol. 2011. PMID: 21098225 Free PMC article.
-
Efficient cross-presentation by heat shock protein 90-peptide complex-loaded dendritic cells via an endosomal pathway.J Immunol. 2007 Aug 1;179(3):1803-13. doi: 10.4049/jimmunol.179.3.1803. J Immunol. 2007. PMID: 17641047
-
Activation of dendritic cells that cross-present tumor-derived antigen licenses CD8+ CTL to cause tumor eradication.J Immunol. 2004 Dec 1;173(11):6753-9. doi: 10.4049/jimmunol.173.11.6753. J Immunol. 2004. PMID: 15557168
-
Toll-like Receptor 4 Engagement on Dendritic Cells Restrains Phago-Lysosome Fusion and Promotes Cross-Presentation of Antigens.Immunity. 2015 Dec 15;43(6):1087-100. doi: 10.1016/j.immuni.2015.11.006. Immunity. 2015. PMID: 26682983
-
Endocytic Recycling of MHC Class I Molecules in Non-professional Antigen Presenting and Dendritic Cells.Front Immunol. 2019 Jan 7;9:3098. doi: 10.3389/fimmu.2018.03098. eCollection 2018. Front Immunol. 2019. PMID: 30666258 Free PMC article. Review.
Cited by
-
The Vacuolar Pathway in Macrophages Plays a Major Role in Antigen Cross-Presentation Induced by the Pore-Forming Protein Sticholysin II Encapsulated Into Liposomes.Front Immunol. 2018 Nov 5;9:2473. doi: 10.3389/fimmu.2018.02473. eCollection 2018. Front Immunol. 2018. PMID: 30455685 Free PMC article.
-
Activation of the Toll‑like receptor 2 signaling pathway inhibits the proliferation of HCC cells in vitro.Oncol Rep. 2019 Dec;42(6):2267-2278. doi: 10.3892/or.2019.7340. Epub 2019 Sep 27. Oncol Rep. 2019. PMID: 31578587 Free PMC article.
-
Adjuvant for vaccine immunotherapy of cancer--focusing on Toll-like receptor 2 and 3 agonists for safely enhancing antitumor immunity.Cancer Sci. 2015 Dec;106(12):1659-68. doi: 10.1111/cas.12824. Epub 2015 Nov 18. Cancer Sci. 2015. PMID: 26395101 Free PMC article. Review.
-
Cell Signaling Pathways That Regulate Antigen Presentation.J Immunol. 2016 Oct 15;197(8):2971-2979. doi: 10.4049/jimmunol.1600460. J Immunol. 2016. PMID: 27824592 Free PMC article. Review.
-
Human γδ T cells induce CD8+ T cell antitumor responses via antigen-presenting effect through HSP90-MyD88-mediated activation of JNK.Cancer Immunol Immunother. 2023 Jun;72(6):1803-1821. doi: 10.1007/s00262-023-03375-w. Epub 2023 Jan 21. Cancer Immunol Immunother. 2023. PMID: 36680568 Free PMC article.
References
-
- Monu N., Trombetta E. S. 2007. Cross-talk between the endocytic pathway and the endoplasmic reticulum in cross-presentation by MHC class I molecules. Curr. Opin. Immunol. 19: 66–72 - PubMed
-
- Guermonprez P., Saveanu L., Kleijmeer M., Davoust J., Van Endert P., Amigorena S. 2003. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature 425: 397–402 - PubMed
-
- Shen L., Sigal L. J., Boes M., Rock K. L. 2004. Important role of cathepsin S in generating peptides for TAP-independent MHC class I crosspresentation in vivo. Immunity 21: 155–165 - PubMed
-
- Shen L., Rock K. L. 2006. Priming of T cells by exogenous antigen cross-presented on MHC class I molecules. Curr. Opin. Immunol. 18: 85–91 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

