Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood
- PMID: 24683445
- PMCID: PMC3968297
- DOI: 10.3402/jev.v3.23743
Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood
Abstract
Introduction: microRNA (miRNA) are small non-coding RNA species that are transcriptionally processed in the host cell and released extracellularly into the bloodstream. Normally involved in post-transcriptional gene silencing, the deregulation of miRNA has been shown to influence pathogenesis of a number of diseases.
Background: Next-generation deep sequencing (NGS) has provided the ability to profile miRNA in biological fluids making this approach a viable screening tool to detect miRNA biomarkers. However, collection and handling procedures of blood needs to be greatly improved for miRNA analysis in order to reliably detect differences between healthy and disease patients. Furthermore, ribonucleases present in blood can degrade RNA upon collection rendering extracellular miRNA at risk of degradation. These factors have consequently decreased sensitivity and specificity of miRNA biomarker assays.
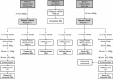
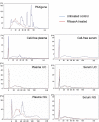
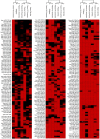
Methods: Here, we use NGS to profile miRNA in various blood components and identify differences in profiles within peripheral blood compared to cell-free plasma or serum and extracellular vesicles known as exosomes. We also analyse and compare the miRNA content in exosomes prepared by ultracentrifugation methods and commercial exosome isolation kits including treating samples with RNaseA.
Conclusion: This study demonstrates that exosomal RNA is protected by RNaseA treatment and that exosomes provide a consistent source of miRNA for disease biomarker detection.
Keywords: deep sequencing; exosomes; microRNA; plasma; serum.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources

