L-type Ca2+ channels in heart and brain
- PMID: 24683526
- PMCID: PMC3968275
- DOI: 10.1002/wmts.102
L-type Ca2+ channels in heart and brain
Abstract
L-type calcium channels (Cav1) represent one of the three major classes (Cav1-3) of voltage-gated calcium channels. They were identified as the target of clinically used calcium channel blockers (CCBs; so-called calcium antagonists) and were the first class accessible to biochemical characterization. Four of the 10 known α1 subunits (Cav1.1-Cav1.4) form the pore of L-type calcium channels (LTCCs) and contain the high-affinity drug-binding sites for dihydropyridines and other chemical classes of organic CCBs. In essentially all electrically excitable cells one or more of these LTCC isoforms is expressed, and therefore it is not surprising that many body functions including muscle, brain, endocrine, and sensory function depend on proper LTCC activity. Gene knockouts and inherited human diseases have allowed detailed insight into the physiological and pathophysiological role of these channels. Genome-wide association studies and analysis of human genomes are currently providing even more hints that even small changes of channel expression or activity may be associated with disease, such as psychiatric disease or cardiac arrhythmias. Therefore, it is important to understand the structure-function relationship of LTCC isoforms, their differential contribution to physiological function, as well as their fine-tuning by modulatory cellular processes.
Figures

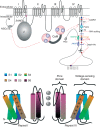
 , proteolytic cleavage site in Cav1.1 and Cav1.2 α1; (sinoatrial node dysfunction and deafness) SANDD, in-frame glycine insertion in SANDD patients. Lower panel: Cartoon of voltage sensing and pore domains of Cav α1 subunits; only two domains (half of the channel) are shown for clarity. Movements of the positively charged S4 helices (which serve as voltage sensors) in response to membrane potential changes are transmitted to the pore domain through the cytoplasmic S4–S5 linkers. S4 movement within the membrane is guided by interactions with negative charges provided by the S1–S3 helices.
, proteolytic cleavage site in Cav1.1 and Cav1.2 α1; (sinoatrial node dysfunction and deafness) SANDD, in-frame glycine insertion in SANDD patients. Lower panel: Cartoon of voltage sensing and pore domains of Cav α1 subunits; only two domains (half of the channel) are shown for clarity. Movements of the positively charged S4 helices (which serve as voltage sensors) in response to membrane potential changes are transmitted to the pore domain through the cytoplasmic S4–S5 linkers. S4 movement within the membrane is guided by interactions with negative charges provided by the S1–S3 helices.

References
-
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. - PubMed
-
- Dolphin AC. Calcium channel auxiliary α2δ and β subunits: trafficking and one step beyond. Nat Rev Neurosci. 2012;13:542–555. - PubMed
-
- Striessnig J, Grabner M, Mitterdorfer J, Hering S, Sinnegger MJ, Glossmann H. Structural basis of drug binding to L calcium channels. Trends Pharmacol Sci. 1998;19:108–115. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
