Automatic prostate MR image segmentation with sparse label propagation and domain-specific manifold regularization
- PMID: 24683995
- PMCID: PMC3974182
- DOI: 10.1007/978-3-642-38868-2_43
Automatic prostate MR image segmentation with sparse label propagation and domain-specific manifold regularization
Abstract
Automatic prostate segmentation in MR images plays an important role in prostate cancer diagnosis. However, there are two main challenges: (1) Large inter-subject prostate shape variations; (2) Inhomogeneous prostate appearance. To address these challenges, we propose a new hierarchical prostate MR segmentation method, with the main contributions lying in the following aspects: First, the most salient features are learnt from atlases based on a subclass discriminant analysis (SDA) method, which aims to find a discriminant feature subspace by simultaneously maximizing the inter-class distance and minimizing the intra-class variations. The projected features, instead of only voxel-wise intensity, will be served as anatomical signature of each voxel. Second, based on the projected features, a new multi-atlases sparse label fusion framework is proposed to estimate the prostate likelihood of each voxel in the target image from the coarse level. Third, a domain-specific semi-supervised manifold regularization method is proposed to incorporate the most reliable patient-specific information identified by the prostate likelihood map to refine the segmentation result from the fine level. Our method is evaluated on a T2 weighted prostate MR image dataset consisting of 66 patients and compared with two state-of-the-art segmentation methods. Experimental results show that our method consistently achieves the highest segmentation accuracies than other methods under comparison.
Figures
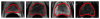




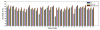
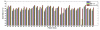

References
-
- Chandra S, Dowling J, Shen K, Raniga P, Pluim J, Greer P, Salvado O, Fripp J. Patient specific prostate segmentation in 3D magnetic resonance images. TMI. 2012;31:1955–1964. - PubMed
-
- Klein S, Heide U, Lips I, Vulpen M, Staring M, Pluim J. Automatic segmentation of the prostate in 3D MR images by atlas matching using localized mutual information. Medical Physics. 2008;35:1407–1417. - PubMed
-
- Toth R, Madabhushi A. Multi-feature landmark-free active appearance models: Application to prostate MRI segmentation. TMI. 2012;31:1638–1650. - PubMed
-
- Martin S, Troccaz J, Daanen V. Automated segmentation of the prostate in 3D MR images using a probabilistic atlas and a spatially constrained deformable model. Medical Physics. 2010;37:1579–1590. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
