Parthanatos: mitochondrial-linked mechanisms and therapeutic opportunities
- PMID: 24684389
- PMCID: PMC3976618
- DOI: 10.1111/bph.12416
Parthanatos: mitochondrial-linked mechanisms and therapeutic opportunities
Abstract
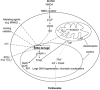
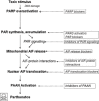
Cells die by a variety of mechanisms. Terminally differentiated cells such as neurones die in a variety of disorders, in part, via parthanatos, a process dependent on the activity of poly (ADP-ribose)-polymerase (PARP). Parthanatos does not require the mediation of caspases for its execution, but is clearly mechanistically dependent on the nuclear translocation of the mitochondrial-associated apoptosis-inducing factor (AIF). The nuclear translocation of this otherwise beneficial mitochondrial protein, occasioned by poly (ADP-ribose) (PAR) produced through PARP overactivation, causes large-scale DNA fragmentation and chromatin condensation, leading to cell death. This review describes the multistep course of parthanatos and its dependence on PAR signalling and nuclear AIF translocation. The review also discusses potential targets in the parthanatos cascade as promising avenues for the development of novel, disease-modifying, therapeutic agents.
Keywords: AIF; PARP-1; cell death; mitochondria; parthanatos; therapy.
© 2013 The British Pharmacological Society.
Figures

References
-
- Abdelkarim GE, Gertz K, Harms C, Katchanov J, Dirnagl U, Szabo C, et al. Protective effects of PJ34, a novel, potent inhibitor of poly(ADP-ribose) polymerase (PARP) in in vitro and in vivo models of stroke. Int J Mol Med. 2001;7:255–260. - PubMed
-
- Ame JC, Apiou F, Jacobson EL, Jacobson MK. Assignment of the poly(ADP-ribose) glycohydrolase gene (PARG) to human chromosome 10q11.23 and mouse chromosome 14B by in situ hybridization. Cytogenet Cell Genet. 1999;85:269–270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

