Oestrogen upregulates the sarcoplasmic reticulum Ca(2+) ATPase pump in coronary arteries
- PMID: 24684418
- PMCID: PMC4036484
- DOI: 10.1111/1440-1681.12233
Oestrogen upregulates the sarcoplasmic reticulum Ca(2+) ATPase pump in coronary arteries
Abstract
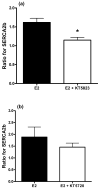
The presence of circulating plasma 17β-oestradiol (E2) is beneficial in women against abnormal vascular tone development, such as coronary arterial vasospasms. Several vascular diseases have demonstrated that increased expression of the sarcoplasmic reticulum Ca(2+) -ATPase pump (SERCA2b) serves to limit the excessive accumulation of intracellular Ca(2+) . Therefore, the hypothesis of the present study was that E2 would increase SERCA2b expression in the coronary vasculature. Coronary arteries were dissected from hearts obtained from mature female pigs. Artery segments were cultured for 24 h in E2 (1 pmol/L or 1 nmol/L) and homogenized for western blot analysis. At 1 nmol/L, E2 induced an approximate 50% increase in immunoreactivity for SERCA2b. In addition, E2 increased the protein expression of the known SERCA regulatory proteins, protein kinase A (PKA) and protein kinase G (PKG). The E2-induced increase in SERCA2b was attenuated when the culture medium was supplemented with the oestrogen receptor (ER) α/β antagonist ICI 182,780 and the PKG antagonist KT5823 (10 μmol/L, 24 h for both). The PKA antagonist (KT5720; 10 μmol/L, 24 h) had no effect on SERCA2b expression. Removal of the endothelium (using a wooden toothpick) from artery segments prior to culture decreased the E2-mediated increase in SERCA2b and PKG expression by 45% and 47%, respectively. Overall, the findings suggest that one of the potential cardiovascular benefits of E2 in women is upregulation of SERCA2b, via activation of the classic ERα and ERβ pathway.
Keywords: Ca2+-ATPase pump; artery; endothelium; oestrogen; protein kinase G; sarcoplasmic reticulum.
© 2014 Wiley Publishing Asia Pty Ltd.
Conflict of interest statement
The authors have no conflict of interest with this study.
Figures





References
-
- Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286:R233–R49. - PubMed
-
- Gilligan DM, Quyyumi AA, Cannon RO., III Effects of physiological levels of estrogen on coronary vasomotor function in postmenopausal women. Circulation. 1994;89:2545–51. - PubMed
-
- Ullrich ND, Koschak A, MacLeod KT. Oestrogen directly inhibits the cardiovascular L-type Ca2+ channel Cav1. 2. Biochemical and Biophysical Research Communications. 2007;361:522–7. - PubMed
-
- Crews JK, Murphy JG, Khalil RA. Gender differences in Ca(2+) entry mechanisms of vasoconstriction in Wistar-Kyoto and spontaneously hypertensive rats. Hypertension. 1999;34:931–6. - PubMed
-
- White RE, Han G, Maunz M, et al. Endothelium-independent effect of estrogen on Ca(2+)-activated K(+) channels in human coronary artery smooth muscle cells. Cardiovasc Res. 2002;53:650–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

