S-nitrosoglutathione reductase deficiency-induced S-nitrosylation results in neuromuscular dysfunction
- PMID: 24684653
- PMCID: PMC4086474
- DOI: 10.1089/ars.2013.5696
S-nitrosoglutathione reductase deficiency-induced S-nitrosylation results in neuromuscular dysfunction
Abstract
Aims: Nitric oxide (NO) production is implicated in muscle contraction, growth and atrophy, and in the onset of neuropathy. However, many aspects of the mechanism of action of NO are not yet clarified, mainly regarding its role in muscle wasting. Notably, whether NO production-associated neuromuscular atrophy depends on tyrosine nitration or S-nitrosothiols (SNOs) formation is still a matter of debate. Here, we aim at assessing this issue by characterizing the neuromuscular phenotype of S-nitrosoglutathione reductase-null (GSNOR-KO) mice that maintain the capability to produce NO, but are unable to reduce SNOs.
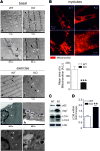
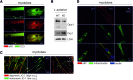
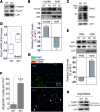
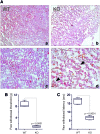
Results: We demonstrate that, without any sign of protein nitration, young GSNOR-KO mice show neuromuscular atrophy due to loss of muscle mass, reduced fiber size, and neuropathic behavior. In particular, GSNOR-KO mice show a significant decrease in nerve axon number, with the myelin sheath appearing disorganized and reduced, leading to a dramatic development of a neuropathic phenotype. Mitochondria appear fragmented and depolarized in GSNOR-KO myofibers and myotubes, conditions that are reverted by N-acetylcysteine treatment. Nevertheless, although atrogene transcription is induced, and bulk autophagy activated, no removal of damaged mitochondria is observed. These events, alongside basal increase of apoptotic markers, contribute to persistence of a neuropathic and myopathic state.
Innovation: Our study provides the first evidence that GSNOR deficiency, which affects exclusively SNOs reduction without altering nitrotyrosine levels, results in a clinically relevant neuromuscular phenotype.
Conclusion: These findings provide novel insights into the involvement of GSNOR and S-nitrosylation in neuromuscular atrophy and neuropathic pain that are associated with pathological states; for example, diabetes and cancer.
Figures





References
-
- Allaire J, Maltais F, LeBlanc P, Simard PM, Whittom F, Doyon JF, Simard C, and Jobin J. Lipofuscin accumulation in the vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Muscle Nerve 25: 383–389, 2002 - PubMed
-
- Barreiro E, Comtois AS, Gea J, Laubach VE, and Hussain SN. Protein tyrosine nitration in the ventilatory muscles: role of nitric oxide synthases. Am J Respir Cell Mol Biol 26: 438–446, 2002 - PubMed
-
- Beal MF, Ferrante RJ, Browne SE, Matthews RT, Kowall NW, and Brown RH., Jr Increased 3-nitrotyrosine in both sporadic and familial amyotrophic lateral sclerosis. Ann Neurol 42: 644–654, 1997 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
