ERK/MAPK regulates ERRγ expression, transcriptional activity and receptor-mediated tamoxifen resistance in ER+ breast cancer
- PMID: 24684682
- PMCID: PMC4079056
- DOI: 10.1111/febs.12797
ERK/MAPK regulates ERRγ expression, transcriptional activity and receptor-mediated tamoxifen resistance in ER+ breast cancer
Abstract
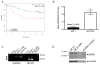
Selective estrogen receptor modulators such as tamoxifen (TAM) significantly improve breast cancer-specific survival for women with estrogen receptor-positive (ER+) disease. However, resistance to TAM remains a major clinical problem. The resistant phenotype is usually not driven by loss or mutation of the estrogen receptor; instead, changes in multiple proliferative and/or survival pathways over-ride the inhibitory effects of TAM. Estrogen-related receptor γ (ERRγ) is an orphan member of the nuclear receptor superfamily that promotes TAM resistance in ER+ breast cancer cells. This study sought to clarify the mechanism(s) by which this orphan nuclear receptor is regulated, and hence affects TAM resistance. mRNA and protein expression/phosphorylation were monitored by RT-PCR and western blotting, respectively. Site-directed mutagenesis was used to disrupt consensus extracellular signal-regulated kinase (ERK) target sites. Cell proliferation and cell-cycle progression were measured by flow cytometric methods. ERRγ transcriptional activity was assessed by dual-luciferase promoter-reporter assays. We show that ERRγ protein levels are affected by the activation state of ERK/mitogen-activated protein kinase, and mutation of consensus ERK target sites impairs ERRγ-driven transcriptional activity and TAM resistance. These findings shed new light on the functional significance of ERRγ in ER+ breast cancer, and are the first to demonstrate a role for kinase regulation of this orphan nuclear receptor.
Keywords: ER+ breast cancer; ERK/MAPK; estrogen-related receptor γ; tamoxifen; transcription.
© 2014 FEBS.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

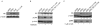
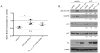
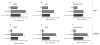
References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R (EBCTCG) EBCTCG. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–84. - PMC - PubMed
-
- Kaufmann M, Pusztai L, Members BEP. Use of standard markers and incorporation of molecular markers into breast cancer therapy: Consensus recommendations from an International Expert Panel. Cancer. 2011;117:1575–82. - PubMed
-
- Musgrove E, Sutherland R. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9:631–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

