Can an alert in primary care electronic medical records increase participation in a population-based screening programme for colorectal cancer? COLO-ALERT, a randomised clinical trial
- PMID: 24685117
- PMCID: PMC3976172
- DOI: 10.1186/1471-2407-14-232
Can an alert in primary care electronic medical records increase participation in a population-based screening programme for colorectal cancer? COLO-ALERT, a randomised clinical trial
Abstract
Background: Colorectal cancer is an important public health problem in Spain. Over the last decade, several regions have carried out screening programmes, but population participation rates remain below recommended European goals. Reminders on electronic medical records have been identified as a low-cost and high-reach strategy to increase participation. Further knowledge is needed about their effect in a population-based screening programme. The main aim of this study is to evaluate the effectiveness of an electronic reminder to promote the participation in a population-based colorectal cancer screening programme. Secondary aims are to learn population's reasons for refusing to take part in the screening programme and to find out the health professionals' opinion about the official programme implementation and on the new computerised tool.
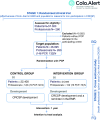
Methods/design: This is a parallel randomised trial with a cross-sectional second stage.
Participants: all the invited subjects to participate in the public colorectal cancer screening programme that includes men and women aged between 50-69, allocated to the eleven primary care centres of the study and all their health professionals. The randomisation unit will be the primary care physician. The intervention will consist of activating an electronic reminder, in the patient's electronic medical record, in order to promote colorectal cancer screening, during a synchronous medical appointment, throughout the year that the intervention takes place. A comparison of the screening rates will then take place, using the faecal occult blood test of the patients from the control and the intervention groups. We will also take a questionnaire to know the opinions of the health professionals. The main outcome is the screening status at the end of the study. Data will be analysed with an intention-to-treat approach.
Discussion: We expect that the introduction of specific reminders in electronic medical records, as a tool to facilitate and encourage direct referral by physicians and nurse practitioners to perform colorectal cancer screening will mean an increase in participation of the target population. The introduction of this new software tool will have good acceptance and increase compliance with recommendations from health professionals.
Trial registration: Clinical Trials.gov identifier NCT01877018.
Figures
References
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v2.0, Cancer Incidence and Mortality Worldwide. IARC Cancer Base No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer; 2010. Available from: [ http://globocan.iarc.fr], accessed on 11/05/2013]
-
- Chirlaque MD, Salmerón D, Ardanaz E, Galceran J, Martínez R, Marcos-Gragera R, Sánchez MJ, Mateos A, Torrella A, Capocaccia R, Navarro C. Cancer survival in Spain: estimate for nine major cancers. Ann Oncol. 2010;21(Suppl 3):iii21–iii29. doi: 10.1093/annonc/mdq082. - PubMed
-
- Morillas JD, Castells A, Oriol I, Pastor A, Pérez-Segura P, Echevarría JM, Caballero B, González-Navarro A, Bandrés F, Brullet E, Iniesta A, Carballo F, Bouzas R, Ariza A, Ibisate A, García-Alfonso P, Escudero B, Camacho S, Fernández-Marcos A, González T, Quintero E, Lanas A, Marzo M, Mascort J, Andréu M, Cerezo L, Vázquez-Sequeiros E, Borrás JM, Salas D, Ascunce N. en representación de la Alianza para la Prevención del Cáncer de Colon en España et al.Alianza para la prevención del cáncer de colon en España: un compromiso cívico con la sociedad. Gastroenterol Hepatol. 2012;35(3):109–128. doi: 10.1016/j.gastrohep.2012.01.002. - DOI - PubMed
-
- Wilson JMG, Junger G. Principles and Practice of Screening for Disease. Geneve: World Health Organization; 1968.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

