Molecular imaging for assessment of mesenchymal stem cells mediated breast cancer therapy
- PMID: 24685267
- PMCID: PMC4414495
- DOI: 10.1016/j.biomaterials.2014.03.014
Molecular imaging for assessment of mesenchymal stem cells mediated breast cancer therapy
Abstract
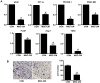
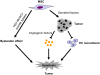
The tumor tropism of mesenchymal stem cells (MSCs) makes them an excellent delivery vehicle used in anticancer therapy. However, the exact mechanisms of MSCs involved in tumor microenvironment are still not well defined. Molecular imaging technologies with the versatility in monitoring the therapeutic effects, as well as basic molecular and cellular processes in real time, offer tangible options to better guide MSCs mediated cancer therapy. In this study, an in situ breast cancer model was developed with MDA-MB-231 cells carrying a reporter system encoding a double fusion (DF) reporter gene consisting of firefly luciferase (Fluc) and enhanced green fluorescent protein (eGFP). In mice breast cancer model, we injected human umbilical cord-derived MSCs (hUC-MSCs) armed with a triple fusion (TF) gene containing the herpes simplex virus truncated thymidine kinase (HSV-ttk), renilla luciferase (Rluc) and red fluorescent protein (RFP) into tumor on day 13, 18, 23 after MDA-MB-231 cells injection. Bioluminescence imaging of Fluc and Rluc provided the real time monitor of tumor cells and hUC-MSCs simultaneously. We found that tumors were significantly inhibited by hUC-MSCs administration, and this effect was enhanced by ganciclovir (GCV) application. To further demonstrate the effect of hUC-MSCs on tumor cells in vivo, we employed the near infrared (NIR) imaging and the results showed that hUC-MSCs could inhibit tumor angiogenesis and increased apoptosis to a certain degree. In conclusion, hUC-MSCs can inhibit breast cancer progression by inducing tumor cell death and suppressing angiogenesis. Moreover, molecular imaging is an invaluable tool in tracking cell delivery and tumor response to hUC-MSCs therapies as well as cellular and molecular processes in tumor.
Keywords: Bioluminescence imaging (BLI); Cancer therapy; Mesenchymal stem cells (MSCs); Molecular imaging; Near infrared (NIF) imaging.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures



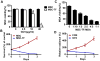

References
-
- Kidd S, Caldwell L, Dietrich M, Samudio I, Spaeth EL, Watson K, et al. Mesenchymal stromal cells alone or expressing interferon-β suppress pancreatic tumors in vivo, an effect countered by anti-inflammatory treatment. Cytotherapy. 2010;12:615–25. - PubMed
-
- Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–18. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

