Coinfection of tick cell lines has variable effects on replication of intracellular bacterial and viral pathogens
- PMID: 24685441
- PMCID: PMC4058533
- DOI: 10.1016/j.ttbdis.2014.01.010
Coinfection of tick cell lines has variable effects on replication of intracellular bacterial and viral pathogens
Abstract
Ticks transmit various human and animal microbial pathogens and may harbour more than one pathogen simultaneously. Both viruses and bacteria can trigger, and may subsequently suppress, vertebrate host and arthropod vector anti-microbial responses. Microbial coinfection of ticks could lead to an advantage or disadvantage for one or more of the microorganisms. In this preliminary study, cell lines derived from the ticks Ixodes scapularis and Ixodes ricinus were infected sequentially with 2 arthropod-borne pathogens, Borrelia burgdorferi s.s., Ehrlichia ruminantium, or Semliki Forest virus (SFV), and the effect of coinfection on the replication of these pathogens was measured. Prior infection of tick cell cultures with the spirochaete B. burgdorferi enhanced subsequent replication of the rickettsial pathogen E. ruminantium whereas addition of spirochaetes to cells infected with E. ruminantium had no effect on growth of the latter. Both prior and subsequent presence of B. burgdorferi also had a positive effect on SFV replication. Presence of E. ruminantium or SFV had no measurable effect on B. burgdorferi growth. In tick cells infected first with E. ruminantium and then with SFV, virus replication was significantly higher across all time points measured (24, 48, 72h post infection), while presence of the virus had no detectable effect on bacterial growth. When cells were infected first with SFV and then with E. ruminantium, there was no effect on replication of either pathogen. The results of this preliminary study indicate that interplay does occur between different pathogens during infection of tick cells. Further study is needed to determine if this results from direct pathogen-pathogen interaction or from effects on host cell defences, and to determine if these observations also apply in vivo in ticks. If presence of one pathogen in the tick vector results in increased replication of another, this could have implications for disease transmission and incidence.
Keywords: Borrelia; Coinfection; Ehrlichia; Ixodes spp.; Semliki Forest virus; Tick cell line.
Copyright © 2014 Elsevier GmbH. All rights reserved.
Figures
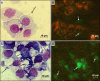
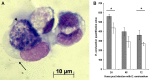

References
-
- Alekseev A.N., Semenov A.V., Dubinina H.V. Evidence of Babesia microti infection in multi-infected Ixodes persulcatus ticks in Russia. Exp. Appl. Acarol. 2003;29:345–353. - PubMed
-
- Ayllon N., Villar M., Busby A.T., Kocan K.M., Blouin E.F., Bonzon-Kulichenko E., Galindo R.C., Mangold A.T., Alberdi P., Perez de la Lastra J.M., Vazquez J., de la Fuente J. Anaplasma phagocytophilum inhibits apoptosis and promotes cytoskeleton rearrangement for infection of tick cells. Infect. Immun. 2013;81:2415–2425. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

