Randomized Phase II Trial of Irinotecan/Docetaxel or Irinotecan/Docetaxel Plus Cetuximab for Metastatic Pancreatic Cancer: An Eastern Cooperative Oncology Group Study
- PMID: 24685886
- PMCID: PMC4177955
- DOI: 10.1097/COC.0000000000000068
Randomized Phase II Trial of Irinotecan/Docetaxel or Irinotecan/Docetaxel Plus Cetuximab for Metastatic Pancreatic Cancer: An Eastern Cooperative Oncology Group Study
Abstract
Objectives: The primary objective was to determine the response rate in patients with metastatic pancreatic cancer treated in first line with irinotecan/docetaxel combination (Arm A) or with irinotecan/docetaxel/cetuximab combination (Arm B). Secondary endpoints were progression-free survival (PFS), overall survival (OS), toxicity, and the rate of thromboembolic events with prophylactic enoxaparin sodium.
Patients and methods: Patients were eligible who had measurable, metastatic adenocarcinoma of the pancreas, and normal bilirubin. All patients received anticoagulation. Docetaxel (35 mg/m) and irinotecan (50 mg/m) were administered once a week for 4 weeks followed by 2 weeks rest (Arm A) alone or with the addition of cetuximab (Arm B). The primary endpoint was response rate.
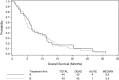
Results: A total of 87 eligible patients were enrolled and treated. Grade 3/4 toxicity was observed in 74% of patients in Arm A and 76% in Arm B. The principal grade 3/4 toxicity was diarrhea. Response rates were 4.5% in Arm A and 7% in Arm B. Median PFS and OS were 3.9 and 6.5 months in Arm A and 4.5 and 5.4 months in Arm B.
Conclusions: Docetaxel/irinotecan combination is associated with considerable toxicity. Objective responses were infrequent and addition of cetuximab in an unselected population was not beneficial, but PFS and OS were comparable with those achieved with other regimens. Docetaxel/irinotecan therapy is active in metastatic pancreatic cancer.
Conflict of interest statement
Dr Burtness reports having served as a consultant for Bristol-Myers Squibb, Sanofi-Aventis, Imclone, Lilly and Pharmacia. Dr Berlin reports having served as a consultant for Imclone and Lilly. Dr. Mitchell reports having served as a consultant for Sanofi-Aventis. Dr Benson reports having served as a consultant for Imclone, Lilly, Bristol-Myers Squibb, and Sanofi-Aventis. The remaining authors declare no conflicts of interest.
References
-
- American Cancer Society. Cancer Facts and Figures, 2013. Available at: http://www.cancer.org. Accessed October 15, 2013.
-
- Burris HA, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. - PubMed
-
- Tempero M, Plunkett W, Ruiz Van Haperen V, et al. Randomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol. 2003;21:3402–3408. - PubMed
-
- Giovannetti E, Mey V, Nannizzi S, et al. Pharmacogenetics of anticancer drug sensitivity in pancreatic cancer. Mol Cancer Ther. 2006;5:1387–1395. - PubMed
-
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical