Exosome-mediated delivery of functionally active miRNA-155 inhibitor to macrophages
- PMID: 24685946
- PMCID: PMC4180003
- DOI: 10.1016/j.nano.2014.03.014
Exosome-mediated delivery of functionally active miRNA-155 inhibitor to macrophages
Abstract
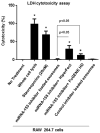
Exosomes, membranous nanovesicles, naturally carry bio-macromolecules and play pivotal roles in both physiological intercellular crosstalk and disease pathogenesis. Here, we showed that B cell-derived exosomes can function as vehicles to deliver exogenous miRNA-155 mimic or inhibitor into hepatocytes or macrophages, respectively. Stimulation of B cells significantly increased exosome production. Unlike in parental cells, baseline level of miRNA-155 was very low in exosomes derived from stimulated B cells. Exosomes loaded with a miRNA-155 mimic significantly increased miRNA-155 levels in primary mouse hepatocytes and the liver of miRNA-155 knockout mice. Treatment of RAW macrophages with miRNA-155 inhibitor loaded exosomes resulted in statistically significant reduction in LPS-induced TNFα production and partially prevented LPS-induced decrease in SOCS1 mRNA levels. Furthermore, exosome-mediated miRNA-155 inhibitor delivery resulted in functionally more efficient inhibition and less cellular toxicity compared to conventional transfection methods. Similar approaches could be useful in modification of target biomolecules in vitro and in vivo. From the clinical editor: In this study, exosome-based delivery of miRNA-155 mimicker or inhibitor was found to have significant biological response in hepatocytes and macrophages. Exosome-based approaches may be useful in the modification of other target biomolecules.
Keywords: Exosomes; Macrophages; RNAs delivery; TNFα; miRNA-155.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


References
-
- EL Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–57. - PubMed
-
- Simpson RJ, Mathivanan S. Extracellular microvesicles: the need for internationally recognised nomenclature and stringent purification criteria. J Proteomics Bioinform. 2012;5:ii–ii.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

