Vitamin d deficiency reduces the immune response, phagocytosis rate, and intracellular killing rate of microglial cells
- PMID: 24686054
- PMCID: PMC4019194
- DOI: 10.1128/IAI.01814-14
Vitamin d deficiency reduces the immune response, phagocytosis rate, and intracellular killing rate of microglial cells
Abstract
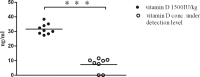
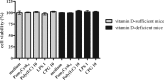
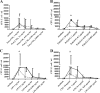
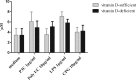
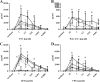
Meningitis and meningoencephalitis caused by Escherichia coli are associated with high rates of mortality and neurological sequelae. A high prevalence of neurological disorders has been observed in geriatric populations at risk of hypovitaminosis D. Vitamin D has potent effects on human immunity, including induction of antimicrobial peptides (AMPs) and suppression of T-cell proliferation, but its influence on microglial cells is unknown. The purpose of the present study was to determine the effects of vitamin D deficiency on the phagocytosis rate, intracellular killing, and immune response of murine microglial cultures after stimulation with the Toll-like receptor (TLR) agonists tripalmitoyl-S-glyceryl-cysteine (TLR1/2), poly(I·C) (TLR3), lipopolysaccharide (TLR4), and CpG oligodeoxynucleotide (TLR9). Upon stimulation with high concentrations of TLR agonists, the release of tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) was decreased in vitamin D-deficient compared to that in vitamin D-sufficient microglial cultures. Phagocytosis of E. coli K1 after stimulation of microglial cells with high concentrations of TLR3, -4, and -9 agonists and intracellular killing of E. coli K1 after stimulation with high concentrations of all TLR agonists were lower in vitamin D-deficient microglial cells than in the respective control cells. Our observations suggest that vitamin D deficiency may impair the resistance of the brain against bacterial infections.
Figures
Similar articles
-
Activin A increases phagocytosis of Escherichia coli K1 by primary murine microglial cells activated by toll-like receptor agonists.J Neuroinflammation. 2018 Jun 7;15(1):175. doi: 10.1186/s12974-018-1209-2. J Neuroinflammation. 2018. PMID: 29880000 Free PMC article.
-
Toll-like receptor prestimulation increases phagocytosis of Escherichia coli DH5alpha and Escherichia coli K1 strains by murine microglial cells.Infect Immun. 2009 Jan;77(1):557-64. doi: 10.1128/IAI.00903-08. Epub 2008 Nov 3. Infect Immun. 2009. PMID: 18981243 Free PMC article.
-
Toll-like receptor stimulation increases phagocytosis of Cryptococcus neoformans by microglial cells.J Neuroinflammation. 2013 Jun 5;10:71. doi: 10.1186/1742-2094-10-71. J Neuroinflammation. 2013. PMID: 23738865 Free PMC article.
-
The role of vitamin D deficiency in sepsis and potential therapeutic implications.J Infect. 2011 Nov;63(5):321-6. doi: 10.1016/j.jinf.2011.07.002. Epub 2011 Jul 13. J Infect. 2011. PMID: 21777617 Review.
-
Vitamin D and Streptococci: The Interface of Nutrition, Host Immune Response, and Antimicrobial Activity in Response to Infection.ACS Infect Dis. 2020 Dec 11;6(12):3131-3140. doi: 10.1021/acsinfecdis.0c00666. Epub 2020 Nov 10. ACS Infect Dis. 2020. PMID: 33170652 Free PMC article. Review.
Cited by
-
Topical Vitamin D Prevents Bone Loss and Inflammation in a Mouse Model.J Dent Res. 2024 Aug;103(9):908-915. doi: 10.1177/00220345241259417. Epub 2024 Aug 5. J Dent Res. 2024. PMID: 39104028 Free PMC article.
-
Antimicrobial and Immune-Modulatory Effects of Vitamin D Provide Promising Antibiotics-Independent Approaches to Tackle Bacterial Infections - Lessons Learnt from a Literature Survey.Eur J Microbiol Immunol (Bp). 2019 Aug 13;9(3):80-87. doi: 10.1556/1886.2019.00014. eCollection 2019 Oct 3. Eur J Microbiol Immunol (Bp). 2019. PMID: 31662886 Free PMC article. Review.
-
Serious vitamin D deficiency in healthcare workers during the COVID-19 pandemic.BMJ Nutr Prev Health. 2022 Jan 4;5(1):134-136. doi: 10.1136/bmjnph-2021-000364. eCollection 2022. BMJ Nutr Prev Health. 2022. PMID: 35814723 Free PMC article. No abstract available.
-
Vitamin D role in hepatitis B: focus on immune system and genetics mechanism.Heliyon. 2022 Nov 15;8(11):e11569. doi: 10.1016/j.heliyon.2022.e11569. eCollection 2022 Nov. Heliyon. 2022. PMID: 36411916 Free PMC article. Review.
-
Opposing effects of alcohol on the immune system.Prog Neuropsychopharmacol Biol Psychiatry. 2016 Feb 4;65:242-51. doi: 10.1016/j.pnpbp.2015.09.001. Epub 2015 Sep 14. Prog Neuropsychopharmacol Biol Psychiatry. 2016. PMID: 26375241 Free PMC article. Review.
References
-
- Gloth FM, III, Gundberg CM, Hollis BW, Haddad JG, Jr, Tobin JD. 1995. Vitamin D deficiency in homebound elderly persons. JAMA 21:1683–1686 - PubMed
-
- Flicker L, MacInnis RJ, Stein MS, Scherer SC, Mead KE, Nowson CA, Thomas J, Lowndes C, Hopper JL, Wark JD. 2005. Should older people in residential care receive vitamin D to prevent falls? Results of a randomized trial. J. Am. Geriatr. Soc. 53:1881–1888. 10.1111/j.1532-5415.2005.00468.x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

