Morphological and functional remodelling of the neuromuscular junction by skeletal muscle PGC-1α
- PMID: 24686533
- PMCID: PMC4846352
- DOI: 10.1038/ncomms4569
Morphological and functional remodelling of the neuromuscular junction by skeletal muscle PGC-1α
Abstract
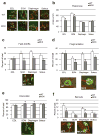
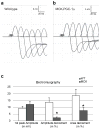
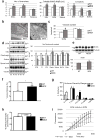
The neuromuscular junction (NMJ) exhibits high morphological and functional plasticity. In the mature muscle, the relative levels of physical activity are the major determinants of NMJ function. Classically, motor neuron-mediated activation patterns of skeletal muscle have been thought of as the major drivers of NMJ plasticity and the ensuing fibre-type determination in muscle. Here we use muscle-specific transgenic animals for the peroxisome proliferator-activated receptor γ co-activator 1α (PGC-1α) as a genetic model for trained mice to elucidate the contribution of skeletal muscle to activity-induced adaptation of the NMJ. We find that muscle-specific expression of PGC-1α promotes a remodelling of the NMJ, even in the absence of increased physical activity. Importantly, these plastic changes are not restricted to post-synaptic structures, but extended to modulation of presynaptic cell morphology and function. Therefore, our data indicate that skeletal muscle significantly contributes to the adaptation of the NMJ subsequent to physical activity.
Conflict of interest statement
None of the authors has competing financial interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

