Disruption of mammalian target of rapamycin complex 1 in macrophages decreases chemokine gene expression and atherosclerosis
- PMID: 24687132
- PMCID: PMC4058053
- DOI: 10.1161/CIRCRESAHA.114.302313
Disruption of mammalian target of rapamycin complex 1 in macrophages decreases chemokine gene expression and atherosclerosis
Abstract
Rationale: The mammalian target of rapamycin complex 1 inhibitor, rapamycin, has been shown to decrease atherosclerosis, even while increasing plasma low-density lipoprotein levels. This suggests an antiatherogenic effect possibly mediated by the modulation of inflammatory responses in atherosclerotic plaques.
Objective: Our aim was to assess the role of macrophage mammalian target of rapamycin complex 1 in atherogenesis.
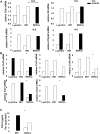
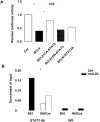
Methods and results: We transplanted bone marrow from mice in which a key mammalian target of rapamycin complex 1 adaptor, regulatory-associated protein of mTOR, was deleted in macrophages by Cre/loxP recombination (Mac-Rap(KO) mice) into Ldlr(-/-) mice and then fed them the Western-type diet. Atherosclerotic lesions from Mac-Rap(KO) mice showed decreased infiltration of macrophages, lesion size, and chemokine gene expression compared with control mice. Treatment of macrophages with minimally modified low-density lipoprotein resulted in increased levels of chemokine mRNAs and signal transducer and activator of transcription (STAT) 3 phosphorylation; these effects were reduced in Mac-Rap(KO) macrophages. Although wild-type and Mac-Rap(KO) macrophages showed similar STAT3 phosphorylation on Tyr705, Mac-Rap(KO) macrophages showed decreased STAT3Ser727 phosphorylation in response to minimally modified low-density lipoprotein treatment and decreased Ccl2 promoter binding of STAT3.
Conclusions: The results demonstrate cross-talk between nutritionally induced mammalian target of rapamycin complex 1 signaling and minimally modified low-density lipoprotein-mediated inflammatory signaling via combinatorial phosphorylation of STAT3 in macrophages, leading to increased STAT3 activity on the chemokine (C-C motif) ligand 2 (monocyte chemoattractant protein 1) promoter with proatherogenic consequences.
Keywords: atherosclerosis; macrophages; mammalian target of rapamycin complex 1.
Figures



References
-
- Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K. Raptor, a binding partner of target of rapamycin (tor), mediates tor action. Cell. 2002;110:177–189. - PubMed
-
- Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Mtor interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. - PubMed
-
- Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mtorc components raptor, rictor, or mlst8 reveals that mtorc2 is required for signaling to akt-foxo and pkcalpha, but not s6k1. Dev Cell. 2006;11:859–871. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

