Sleep and circadian rhythm regulation in early Parkinson disease
- PMID: 24687146
- PMCID: PMC4119609
- DOI: 10.1001/jamaneurol.2014.65
Sleep and circadian rhythm regulation in early Parkinson disease
Abstract
Importance: Sleep disturbances are recognized as a common nonmotor complaint in Parkinson disease but their etiology is poorly understood.
Objective: To define the sleep and circadian phenotype of patients with early-stage Parkinson disease.
Design, setting, and participants: Initial assessment of sleep characteristics in a large population-representative incident Parkinson disease cohort (N=239) at the University of Cambridge, England, followed by further comprehensive case-control sleep assessments in a subgroup of these patients (n=30) and matched controls (n=15).
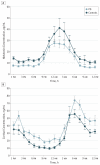
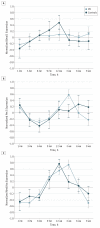
Main outcomes and measures: Sleep diagnoses and sleep architecture based on polysomnography studies, actigraphy assessment, and 24-hour analyses of serum cortisol, melatonin, and peripheral clock gene expression (Bmal1, Per2, and Rev-Erbα).
Results: Subjective sleep complaints were present in almost half of newly diagnosed patients and correlated significantly with poorer quality of life. Patients with Parkinson disease exhibited increased sleep latency (P = .04), reduced sleep efficiency (P = .008), and reduced rapid eye movement sleep (P = .02). In addition, there was a sustained elevation of serum cortisol levels, reduced circulating melatonin levels, and altered Bmal1 expression in patients with Parkinson disease compared with controls.
Conclusions and relevance: Sleep dysfunction seen in early Parkinson disease may reflect a more fundamental pathology in the molecular clock underlying circadian rhythms.
Figures
Comment in
-
The central clock in patients with Parkinson disease.JAMA Neurol. 2014 Nov;71(11):1455-6. doi: 10.1001/jamaneurol.2014.2708. JAMA Neurol. 2014. PMID: 25383775 No abstract available.
-
The central clock in patients with Parkinson disease--reply.JAMA Neurol. 2014 Nov;71(11):1456-7. doi: 10.1001/jamaneurol.2014.2711. JAMA Neurol. 2014. PMID: 25383776 Free PMC article. No abstract available.
References
-
- Barone P, Antonini A, Colosimo C, et al. PRIAMO study group The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord. 2009;24(11):1641–1649. - PubMed
-
- Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8(5):464–474. - PubMed
-
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. - PubMed
-
- Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4(8):649–661. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

