Role of public standards in the safety and efficacy of biologic medicines
- PMID: 24687209
- PMCID: PMC4012053
- DOI: 10.1208/s12248-014-9586-7
Role of public standards in the safety and efficacy of biologic medicines
Abstract
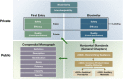
In this report, we emphasize the importance of public monographs with reference materials, coupled with careful process and change control and attention to GMPs, as a means of advancing access to good quality, safe, and effective medicines, with emphasis on available and incoming biologic medicines. With adequate control of articles covered by a monograph, these public standards can form the basis for a global public quality platform that covers reference products, non-interchangeable reference products, biosimilars, and interchangeable biosimilars. Working collaboratively with all stakeholders, new approaches allow these public standards to emerge nationally and globally in a timely way. Yet, there are increasing limitations in the availability of public standards for biologic medicines, which may reverse many decades of progress. Solutions are considered in this report.
Figures


References
-
- NIBSC. http://www.nibsc.org/products/reference_standards/biological_reference_m... Accessed 17 Sep 2013.
-
- Fields B. From rabbits to the League of Nations: early standardization of the insulin unit. The Pharos. 2011;74(1):28–33. - PubMed
-
- WHO. http://www.who.int/biologicals/reference_preparations/en/. Accessed 17 Sep 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

