Transport and diffusion of Tau protein in neurons
- PMID: 24687422
- PMCID: PMC11113808
- DOI: 10.1007/s00018-014-1610-7
Transport and diffusion of Tau protein in neurons
Abstract
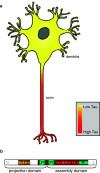
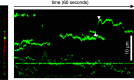
In highly polarized and elongated cells such as neurons, Tau protein must enter and move down the axon to fulfill its biological task of stabilizing axonal microtubules. Therefore, cellular systems for distributing Tau molecules are needed. This review discusses different mechanisms that have been proposed to contribute to the dispersion of Tau molecules in neurons. They include (1) directed transport along microtubules as cargo of tubulin complexes and/or motor proteins, (2) diffusion, either through the cytosolic space or along microtubules, and (3) mRNA-based mechanisms such as transport of Tau mRNA into axons and local translation. Diffusion along the microtubule lattice or through the cytosol appear to be the major mechanisms for axonal distribution of Tau protein in the short-to-intermediate range over distances of up to a millimetre. The high diffusion coefficients ensure that Tau can distribute evenly throughout the axonal volume as well as along microtubules. Motor protein-dependent transport of Tau dominates over longer distances and time scales. At low near-physiological levels, Tau is co-transported along with short microtubules from cell bodies into axons by cytoplasmic dynein and kinesin family members at rates of slow axonal transport.
Figures


References
-
- Ackmann M, Wiech H, Mandelkow E. Nonsaturable binding indicates clustering of tau on the microtubule surface in a paired helical filament-like conformation. J Biol Chem. 2000;275(39):30335–30343. - PubMed
-
- Ahmad FJ, Baas PW. Microtubules released from the neuronal centrosome are transported into the axon. J Cell Sci. 1995;108(Pt 8):2761–2769. - PubMed
-
- Andreadis A. Tau gene alternative splicing: expression patterns, regulation and modulation of function in normal brain and neurodegenerative diseases. Biochim Biophys Acta. 2005;1739(2–3):91–103. - PubMed
-
- Andreadis A, Brown WM, Kosik KS. Structure and novel exons of the human tau gene. Biochemistry. 1992;31(43):10626–10633. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

