Altered pharmacokinetics of piperacillin in febrile neutropenic patients with hematological malignancy
- PMID: 24687508
- PMCID: PMC4068433
- DOI: 10.1128/AAC.02340-14
Altered pharmacokinetics of piperacillin in febrile neutropenic patients with hematological malignancy
Abstract
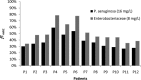
This study assessed the pharmacokinetics and dosing adequacy of piperacillin in febrile neutropenic patients after the first dose. Pharmacokinetic analysis was performed using noncompartmental methods. We observed an elevated volume of distribution (29.7 ± 8.0 liters [mean ± standard deviation]) and clearance (20.2 ± 7.5 liters/h) compared to data from other patient populations. Antibiotic exposure did not consistently result in therapeutic targets. We conclude that alternative dosing strategies guided by therapeutic drug monitoring may be required to optimize exposure.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Lortholary O, Lefort A, Tod M, Chomat AM, Darras-Joly C, Cordonnier C, Club de Reflexion sur les Infections en Onco-Hématologie 2008. Pharmacodynamics and pharmacokinetics of antibacterial drugs in the management of febrile neutropenia. Lancet Infect. Dis. 8:612–620. 10.1016/S1473-3099(08)70228-7 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

