CD4+ T cells enhance the unloaded shortening velocity of airway smooth muscle by altering the contractile protein expression
- PMID: 24687581
- PMCID: PMC4214656
- DOI: 10.1113/jphysiol.2014.270843
CD4+ T cells enhance the unloaded shortening velocity of airway smooth muscle by altering the contractile protein expression
Abstract
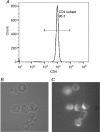
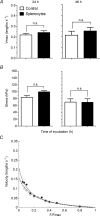
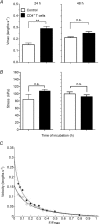
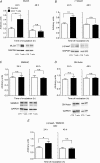
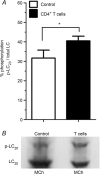
Abundant data indicate that pathogenesis in allergic airways disease is orchestrated by an aberrant T-helper 2 (Th2) inflammatory response. CD4(+) T cells have been localized to airway smooth muscle (ASM) in both human asthmatics and in rodent models of allergic airways disease, where they have been implicated in proliferative responses of ASM. Whether CD4(+) T cells also alter ASM contractility has not been addressed. We established an in vitro system to assess the ability of antigen-stimulated CD4(+) T cells to modify contractile responses of the Brown Norway rat trachealis muscle. Our data demonstrated that the unloaded velocity of shortening (Vmax) of ASM was significantly increased upon 24 h co-incubation with antigen-stimulated CD4(+) T cells, while stress did not change. Enhanced Vmax was dependent upon contact between the CD4(+) T cells and the ASM and correlated with increased levels of the fast (+)insert smooth muscle myosin heavy chain isoform. The levels of myosin light chain kinase and myosin light chain phosphorylation were also increased within the muscle. The alterations in mechanics and in the levels of contractile proteins were transient, both declining to control levels after 48 h of co-incubation. More permanent alterations in muscle phenotype might be attainable when several inflammatory cells and mediators interact together or after repeated antigenic challenges. Further studies will await new tissue culture methodologies that preserve the muscle properties over longer periods of time. In conclusion, our data suggest that inflammatory cells promote ASM hypercontractility in airway hyper-responsiveness and asthma.
© 2014 The Authors. The Journal of Physiology © 2014 The Physiological Society.
Figures


Similar articles
-
Mechanical strain increases velocity and extent of shortening in cultured airway smooth muscle cells.Am J Physiol. 1999 Aug;277(2):L343-8. doi: 10.1152/ajplung.1999.277.2.L343. Am J Physiol. 1999. PMID: 10444529
-
Molecular mechanics of smooth muscle contractile proteins in airway hyperresponsiveness and asthma.Proc Am Thorac Soc. 2008 Jan 1;5(1):40-6. doi: 10.1513/pats.200704-053VS. Proc Am Thorac Soc. 2008. PMID: 18094083 Review.
-
Peripheral Airway Smooth Muscle, but Not the Trachealis, Is Hypercontractile in an Equine Model of Asthma.Am J Respir Cell Mol Biol. 2016 May;54(5):718-27. doi: 10.1165/rcmb.2015-0180OC. Am J Respir Cell Mol Biol. 2016. PMID: 26473389 Free PMC article.
-
Human trachealis and main bronchi smooth muscle are normoresponsive in asthma.Am J Respir Crit Care Med. 2015 Apr 15;191(8):884-93. doi: 10.1164/rccm.201407-1296OC. Am J Respir Crit Care Med. 2015. PMID: 25695616 Free PMC article.
-
Myosin isoforms and functional diversity in vertebrate smooth muscle.Comp Biochem Physiol B Biochem Mol Biol. 1997 May;117(1):51-60. doi: 10.1016/s0305-0491(96)00314-8. Comp Biochem Physiol B Biochem Mol Biol. 1997. PMID: 9180014 Review.
Cited by
-
Tissue-specific expression of myosin phosphatase subunits and isoforms in smooth muscle of mice and humans.Am J Physiol Regul Integr Comp Physiol. 2022 Apr 1;322(4):R281-R291. doi: 10.1152/ajpregu.00196.2021. Epub 2022 Feb 2. Am J Physiol Regul Integr Comp Physiol. 2022. PMID: 35107022 Free PMC article.
-
Treatment with a sphingosine analog after the inception of house dust mite-induced airway inflammation alleviates key features of experimental asthma.Respir Res. 2015 Feb 3;16(1):7. doi: 10.1186/s12931-015-0180-z. Respir Res. 2015. PMID: 25645346 Free PMC article.
-
Airway smooth muscle function in asthma.Front Physiol. 2022 Oct 5;13:993406. doi: 10.3389/fphys.2022.993406. eCollection 2022. Front Physiol. 2022. PMID: 36277199 Free PMC article. Review.
-
Neonatal Streptococcus pneumoniae Pneumonia Induces an Aberrant Airway Smooth Muscle Phenotype and AHR in Mice Model.Biomed Res Int. 2019 Jan 6;2019:1948519. doi: 10.1155/2019/1948519. eCollection 2019. Biomed Res Int. 2019. PMID: 30723734 Free PMC article.
-
Crosstalk between CD4+ T Cells and Airway Smooth Muscle in Pediatric Obesity-related Asthma.Am J Respir Crit Care Med. 2023 Feb 15;207(4):461-474. doi: 10.1164/rccm.202205-0985OC. Am J Respir Crit Care Med. 2023. PMID: 36194662 Free PMC article.
References
-
- Al Heialy S, Risse PA, Zeroual MA, Roman HN, Tsuchiya K, Siddiqui S, Laporte SA, Martin JG. T cell-induced airway smooth muscle cell proliferation via the epidermal growth factor receptor. Am J Respir Cell Mol Biol. 2013;49:563–570. - PubMed
-
- Ammit AJ, Armour CL, Black JL. Smooth-muscle myosin light-chain kinase content is increased in human sensitized airways. Am J Respir Crit Care Med. 2000;161:257–263. - PubMed
-
- Antonissen LA, Mitchell RW, Kroeger EA, Kepron W, Tse KS, Stephens NL. Mechanical alterations of airway smooth muscle in a canine asthmatic model. J Appl Physiol. 1979;46:681–687. - PubMed
-
- Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O'Byrne P, Pedersen SE, Pizzichini E, Sullivan SD, Wenzel SE, Zar HJ. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. - PubMed
-
- Becker AB, Hershkovich J, Simons FER, Simons KJ, Lilley MK, Kepron MW. Development of chronic airway hyperresponsiveness in ragweed-sensitized dogs. J Appl Physiol. 1989;66:2691–2697. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

