Smooth muscle BK channel activity influences blood pressure independent of vascular tone in mice
- PMID: 24687584
- PMCID: PMC4080938
- DOI: 10.1113/jphysiol.2014.272880
Smooth muscle BK channel activity influences blood pressure independent of vascular tone in mice
Abstract
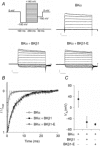
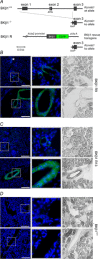
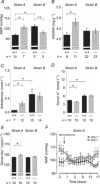
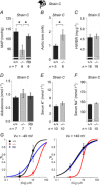
The large conductance voltage- and Ca(2+)-activated K(+) (BK) channel is an important determinant of vascular tone and contributes to blood pressure regulation. Both activities depend on the ancillary BKβ1 subunit. To determine the significance of smooth muscle BK channel activity for blood pressure regulation, we investigated the potential link between changes in arterial tone and altered blood pressure in BKβ1 knockout (BKβ1(-/-)) mice from three different genetically defined strains. While vascular tone was consistently increased in all BKβ1(-/-) mice independent of genetic background, BKβ1(-/-) strains exhibited increased (strain A), unaltered (strain B) or decreased (strain C) mean arterial blood pressures compared to their corresponding BKβ1(+/+) controls. In agreement with previous data on aldosterone regulation by renal/adrenal BK channel function, BKβ1(-/-) strain A mice have increased plasma aldosterone and increased blood pressure. Consistently, blockade of mineralocorticoid receptors by spironolactone treatment reversibly restored the elevated blood pressure to the BKβ1(+/+) strain A level. In contrast, loss of BKβ1 did not affect plasma aldosterone in strain C mice. Smooth muscle-restricted restoration of BKβ1 expression increased blood pressure in BKβ1(-/-) strain C mice, implying that impaired smooth muscle BK channel activity lowers blood pressure in these animals. We conclude that BK channel activity directly affects vascular tone but influences blood pressure independent of this effect via different pathways.
© 2014 The Authors. The Journal of Physiology © 2014 The Physiological Society.
Figures



Similar articles
-
Mice with disrupted BK channel beta1 subunit gene feature abnormal Ca(2+) spark/STOC coupling and elevated blood pressure.Circ Res. 2000 Nov 24;87(11):E53-60. doi: 10.1161/01.res.87.11.e53. Circ Res. 2000. PMID: 11090555
-
Aging influences cerebrovascular myogenic reactivity and BK channel function in a sex-specific manner.Cardiovasc Res. 2020 Jun 1;116(7):1372-1385. doi: 10.1093/cvr/cvz314. Cardiovasc Res. 2020. PMID: 31738403
-
Diabetes downregulates large-conductance Ca2+-activated potassium beta 1 channel subunit in retinal arteriolar smooth muscle.Circ Res. 2007 Mar 16;100(5):703-11. doi: 10.1161/01.RES.0000260182.36481.c9. Epub 2007 Feb 9. Circ Res. 2007. PMID: 17293477 Free PMC article.
-
BK channels and a new form of hypertension.Kidney Int. 2010 Nov;78(10):956-62. doi: 10.1038/ki.2010.272. Epub 2010 Aug 18. Kidney Int. 2010. PMID: 20720523 Free PMC article. Review.
-
Role of BK channels in hypertension and potassium secretion.Curr Opin Nephrol Hypertens. 2011 Sep;20(5):512-7. doi: 10.1097/MNH.0b013e3283488889. Curr Opin Nephrol Hypertens. 2011. PMID: 21670674 Free PMC article. Review.
Cited by
-
Cardiac function modulation depends on the A-kinase anchoring protein complex.J Cell Mol Med. 2019 Nov;23(11):7170-7179. doi: 10.1111/jcmm.14659. Epub 2019 Sep 11. J Cell Mol Med. 2019. PMID: 31512389 Free PMC article. Review.
-
Fluorogenic Green-Inside Red-Outside (GIRO) Labeling Approach Reveals Adenylyl Cyclase-Dependent Control of BKα Surface Expression.Bioconjug Chem. 2015 Sep 16;26(9):1963-71. doi: 10.1021/acs.bioconjchem.5b00409. Epub 2015 Sep 2. Bioconjug Chem. 2015. PMID: 26301573 Free PMC article.
-
Challenges in the Therapeutic Targeting of KCa Channels: From Basic Physiology to Clinical Applications.Int J Mol Sci. 2024 Mar 4;25(5):2965. doi: 10.3390/ijms25052965. Int J Mol Sci. 2024. PMID: 38474212 Free PMC article. Review.
-
Membrane potential and Ca2+ concentration dependence on pressure and vasoactive agents in arterial smooth muscle: A model.J Gen Physiol. 2015 Jul;146(1):79-96. doi: 10.1085/jgp.201511380. J Gen Physiol. 2015. PMID: 26123196 Free PMC article.
-
BK channel β1-subunit deficiency exacerbates vascular fibrosis and remodelling but does not promote hypertension in high-fat fed obesity in mice.J Hypertens. 2015 Aug;33(8):1611-23. doi: 10.1097/HJH.0000000000000590. J Hypertens. 2015. PMID: 26049174 Free PMC article.
References
-
- Amberg GC, Santana LF. Downregulation of the BK channel β1 subunit in genetic hypertension. Circ Res. 2003;93:965–971. - PubMed
-
- Behrens R, Nolting A, Reimann F, Schwarz M, Waldschutz R, Pongs O. hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel beta subunit family. FEBS Lett. 2000;474:99–106. - PubMed
-
- Brenner R, Perez GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. - PubMed
-
- Briet M, Schiffrin EL. Aldosterone: effects on the kidney and cardiovascular system. Nat Rev Nephrol. 2010;6:261–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

