Microenvironment influences vascular differentiation of murine cardiovascular progenitor cells
- PMID: 24687591
- PMCID: PMC7678501
- DOI: 10.1002/jbm.b.33155
Microenvironment influences vascular differentiation of murine cardiovascular progenitor cells
Abstract
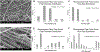
We examined the effects of the microenvironment on vascular differentiation of murine cardiovascular progenitor cells (CPCs). We isolated CPCs and seeded them in culture exposed to the various extracellular matrix (ECM) proteins in both two-dimensional (2D) and 3D culture systems. To better understand the contribution of the microenvironment to vascular differentiation, we analyzed endothelial and smooth muscle cell differentiation at both day 7 and day 14. We found that laminin and vitronectin enhanced vascular endothelial cell differentiation while fibronectin enhanced vascular smooth muscle cell differentiation. We also observed that the effects of the 3D electrospun scaffolds were delayed and not noticeable until the later time point (day 14), which may be due to the amount of time necessary for the cells to migrate to the interior of the scaffold. The study characterized the contributions of both ECM proteins and the addition of a 3D culture system to continued vascular differentiation. Additionally, we demonstrated the capability bioengineer a CPC-derived vascular graft.
Keywords: cell differentiation; extracellular matrix; progenitor cells; scaffolds; stem cells; vascular.
© 2014 Wiley Periodicals, Inc.
Figures



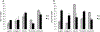


Similar articles
-
Isolation and characterization of a Sca-1+/CD31- progenitor cell lineage derived from mouse heart tissue.BMC Biotechnol. 2014 Aug 9;14:75. doi: 10.1186/1472-6750-14-75. BMC Biotechnol. 2014. PMID: 25106452 Free PMC article.
-
Endothelial and smooth muscle cells derived from human cardiac explants demonstrate angiogenic potential and suitable for design of cell-containing vascular grafts.J Transl Med. 2017 Mar 3;15(1):54. doi: 10.1186/s12967-017-1156-1. J Transl Med. 2017. PMID: 28257636 Free PMC article.
-
Cardiac Adipose-Derived Stem Cells Exhibit High Differentiation Potential to Cardiovascular Cells in C57BL/6 Mice.Stem Cells Transl Med. 2016 Feb;5(2):141-51. doi: 10.5966/sctm.2015-0083. Epub 2015 Dec 18. Stem Cells Transl Med. 2016. PMID: 26683873 Free PMC article.
-
Regulation of the matrix microenvironment for stem cell engineering and regenerative medicine.Ann Biomed Eng. 2011 Apr;39(4):1201-14. doi: 10.1007/s10439-011-0297-2. Epub 2011 Mar 22. Ann Biomed Eng. 2011. PMID: 21424849 Free PMC article. Review.
-
The role of smooth muscle cells in vessel wall pathophysiology and reconstruction using bioactive synthetic polymers.Physiol Res. 2011;60(3):419-37. doi: 10.33549/physiolres.932038. Epub 2011 Mar 14. Physiol Res. 2011. PMID: 21401306 Review.
Cited by
-
Vessel graft fabricated by the on-site differentiation of human mesenchymal stem cells towards vascular cells on vascular extracellular matrix scaffold under mechanical stimulation in a rotary bioreactor.J Mater Chem B. 2019 Apr 28;7(16):2703-2713. doi: 10.1039/c8tb03348j. Epub 2019 Mar 26. J Mater Chem B. 2019. PMID: 32255003 Free PMC article.
-
Fabrication and Characterization of Pectin Hydrogel Nanofiber Scaffolds for Differentiation of Mesenchymal Stem Cells into Vascular Cells.ACS Biomater Sci Eng. 2019 Dec 9;5(12):6511-6519. doi: 10.1021/acsbiomaterials.9b01178. Epub 2019 Nov 12. ACS Biomater Sci Eng. 2019. PMID: 33417803 Free PMC article.
References
-
- Malik S, Wong ND, Franklin SS, Kamath TV, L’Italien GJ, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation 2004;110:1245–1250. - PubMed
-
- Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, Ganiats TG, Goldstein S, Gregoratos G, Jessup ML, Noble RJ, Packer M, Silver MA, Stevenson LW, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Jacobs AK, Hiratzka LF, Russel RO, Smith SC. ACC/AHA Guidelines for the evaluation and management of chronic heart failure in the adult: Executive Summary; A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (committee to revise the 1995 guidelines for the evaluation and management of heart failure): Developed in collaboration with the International Society for Heart and Lung Transplantation, Endorsed by the Heart Failure Society of America. Circulation 2001;104:2996–3007. - PubMed
-
- Ornish D, Brown SE, Scherwitz LW, Armstrong WT, Ports TA, McLanahan SM, Kirkeeide RL, Gould KL, Brand RJ. Can lifestyle changes reverse coronary heart disease? Lancet 1990;336:129–133. - PubMed
-
- Fonarow GC, Gawlinski A, Moughrabi S, Tillisch JH. Improved treatment of coronary heart disease by implementation of a cardiac hospitalization atherosclerosis management program (CHAMP). Am J Cardiol 2001;87:819–822. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

