Pathophysiology of glia in perinatal white matter injury
- PMID: 24687630
- PMCID: PMC4163108
- DOI: 10.1002/glia.22658
Pathophysiology of glia in perinatal white matter injury
Abstract
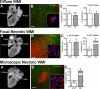
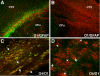
Injury to the preterm brain has a particular predilection for cerebral white matter. White matter injury (WMI) is the most common cause of brain injury in preterm infants and a major cause of chronic neurological morbidity including cerebral palsy. Factors that predispose to WMI include cerebral oxygenation disturbances and maternal-fetal infection. During the acute phase of WMI, pronounced oxidative damage occurs that targets late oligodendrocyte progenitors (pre-OLs). The developmental predilection for WMI to occur during prematurity appears to be related to both the timing of appearance and regional distribution of susceptible pre-OLs that are vulnerable to a variety of chemical mediators including reactive oxygen species, glutamate, cytokines, and adenosine. During the chronic phase of WMI, the white matter displays abberant regeneration and repair responses. Early OL progenitors respond to WMI with a rapid robust proliferative response that results in a several fold regeneration of pre-OLs that fail to terminally differentiate along their normal developmental time course. Pre-OL maturation arrest appears to be related in part to inhibitory factors that derive from reactive astrocytes in chronic lesions. Recent high field magnetic resonance imaging (MRI) data support that three distinct forms of chronic WMI exist, each of which displays unique MRI and histopathological features. These findings suggest the possibility that therapies directed at myelin regeneration and repair could be initiated early after WMI and monitored over time. These new mechanisms of acute and chronic WMI provide access to a variety of new strategies to prevent or promote repair of WMI in premature infants.
Keywords: hyperoxia; hypoxia; hypoxia-ischemia; myelin; oligodendrocyte.
© 2014 Wiley Periodicals, Inc.
Figures

References
-
- (CDC) CfDCaP. Economic costs associated with mental retardation, cerebral palsy, hearing loss and vision impairment--United States, 2003. MMWR Morb Mortal Wkly Rep. 2004;53:57–59. - PubMed
-
- Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124:717–28. - PubMed
-
- Agresti C, D'Urso D, Levi G. Reversible inhibitory effects of interferon-γ and tumor necrosis factor-α on oligodendroglial lineage cell proliferation and differentiation in vitro. Eur J Neurosci. 1996;8:1106–1116. - PubMed
-
- Aguirre A, Dupree JL, Mangin JM, Gallo V. A functional role for EGFR signaling in myelination and remyelination. Nat Neurosci. 2007;10:990–1002. - PubMed
-
- Alix JJ, Fern R. Glutamate receptor-mediated ischemic injury of premyelinated central axons. Ann Neurol. 2009;66:682–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

