Patient-reported outcome results from the open-label phase III AURELIA trial evaluating bevacizumab-containing therapy for platinum-resistant ovarian cancer
- PMID: 24687829
- PMCID: PMC4876313
- DOI: 10.1200/JCO.2013.51.4240
Patient-reported outcome results from the open-label phase III AURELIA trial evaluating bevacizumab-containing therapy for platinum-resistant ovarian cancer
Abstract
Purpose: To determine the effects of bevacizumab on patient-reported outcomes (PROs; secondary end point) in the AURELIA trial.
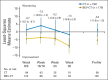
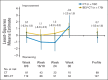
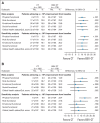
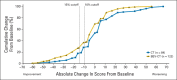
Patients and methods: Patients with platinum-resistant ovarian cancer were randomly assigned to chemotherapy alone (CT) or with bevacizumab (BEV-CT). PROs were assessed using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Ovarian Cancer Module 28 (EORTC QLQ-OV28) and Functional Assessment of Cancer Therapy-Ovarian Cancer symptom index (FOSI) at baseline and every two or three cycles (8/9 weeks) until disease progression. The primary PRO hypothesis was that more patients receiving BEV-CT than CT would achieve at least a 15% (≥ 15-point) absolute improvement on the QLQ-OV28 abdominal/GI symptom subscale (items 31-36) at week 8/9. Patients with missing week 8/9 questionnaires were included as unimproved. Questionnaires from all assessments until disease progression were analyzed using mixed-model repeated-measures (MMRM) analysis. Sensitivity analyses were used to determine the effects of differing assumptions and methods for missing data.
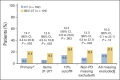
Results: Baseline questionnaires were available from 89% of 361 randomly assigned patients. More BEV-CT than CT patients achieved a ≥ 15% improvement in abdominal/GI symptoms at week 8/9 (primary PRO end point, 21.9% v 9.3%; difference, 12.7%; 95% CI, 4.4 to 20.9; P = .002). MMRM analysis covering all time points also favored BEV-CT (difference, 6.4 points; 95% CI, 1.3 to 11.6; P = .015). More BEV-CT than CT patients achieved ≥ 15% improvement in FOSI at week 8/9 (12.2% v 3.1%; difference, 9.0%; 95% CI, 2.9% to 15.2%; P = .003). Sensitivity analyses gave similar results and conclusions.
Conclusion: Bevacizumab increased the proportion of patients achieving a 15% improvement in patient-reported abdominal/GI symptoms during chemotherapy for platinum-resistant ovarian cancer.
Trial registration: ClinicalTrials.gov NCT00976911.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


Comment in
-
Emerging role for bevacizumab in combination with chemotherapy for patients with platinum-resistant ovarian cancer.J Clin Oncol. 2014 May 1;32(13):1287-9. doi: 10.1200/JCO.2013.54.7299. Epub 2014 Mar 17. J Clin Oncol. 2014. PMID: 24637996 No abstract available.
References
-
- Naumann RW, Coleman RL. Management strategies for recurrent platinum-resistant ovarian cancer. Drugs. 2011;71:1397–1412. - PubMed
-
- Friedlander M, Butow P, Stockler M, et al. Symptom control in patients with recurrent ovarian cancer: Measuring the benefit of palliative chemotherapy in women with platinum refractory/resistant ovarian cancer. Int J Gynecol Cancer. 2009;19(suppl 2):S44–S48. - PubMed
-
- Pujade-Lauraine E, Hilpert F, Weber B, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32:1302–1308. - PubMed
-
- Friedlander M, Trimble E, Tinker A, et al. Clinical trials in recurrent ovarian cancer. Int J Gynecol Cancer. 2011;21:771–775. - PubMed
-
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

