USP28 is recruited to sites of DNA damage by the tandem BRCT domains of 53BP1 but plays a minor role in double-strand break metabolism
- PMID: 24687851
- PMCID: PMC4019063
- DOI: 10.1128/MCB.00197-14
USP28 is recruited to sites of DNA damage by the tandem BRCT domains of 53BP1 but plays a minor role in double-strand break metabolism
Abstract
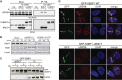
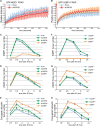
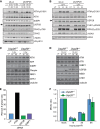
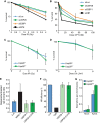
The DNA damage response (DDR) is critical for genome stability and the suppression of a wide variety of human malignancies, including neurodevelopmental disorders, immunodeficiency, and cancer. In addition, the efficacy of many chemotherapeutic strategies is dictated by the status of the DDR. Ubiquitin-specific protease 28 (USP28) was reported to govern the stability of multiple factors that are critical for diverse aspects of the DDR. Here, we examined the effects of USP28 depletion on the DDR in cells and in vivo. We found that USP28 is recruited to double-strand breaks in a manner that requires the tandem BRCT domains of the DDR protein 53BP1. However, we observed only minor DDR defects in USP28-depleted cells, and mice lacking USP28 showed normal longevity, immunological development, and radiation responses. Our results thus indicate that USP28 is not a critical factor in double-strand break metabolism and is unlikely to be an attractive target for therapeutic intervention aimed at chemotherapy sensitization.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
